Article Text
Abstract
Background The effectiveness of intra-articular hyaluronic acid (IAHA) injection for knee osteoarthritis (KOA) is debated.
Objectives To evaluate the effect of IAHA for patients with KOA by analysing data from trials of IAHA versus placebo with low risk of bias, to provide the highest level of evidence.
Methods A systematic review and meta-analysis was conducted. Randomised controlled trials (RCTs) with a low risk of bias (adequate randomisation and concealment and double-blind design) that investigated IAHA versus placebo (saline solution) injection were eligible. The primary efficacy measure was pain intensity and secondary outcome function at 3 months. The treatment effect was summarised with the standardised mean difference (SMD) calculated from differences in means of pain and function measures between treatment and control groups at 3 months. Trials were pooled by a random-effects model with DerSimonian and Laird weights. Statistical heterogeneity was explored by a visual exploration of forest plots and the I2 statistic.
Results A total of eight RCTs (2 199 randomised patients) met our inclusion criteria. IAHA significantly reduced the pain intensity (SMD=−0.21, 95% CI (95% CI) −0.32 to −0.10) and improved function (SMD=−0.12, 95% CI −0.22 to −0.02). Trials showed no heterogeneity.
Conclusions This meta-analysis of high-quality trials of IAHA versus placebo shows that IAHA provides a moderate but real benefit for patients with KOA.
- Osteoarthritis
- Outcomes research
- Treatment
This is an Open Access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Statistics from Altmetric.com
Key messages
What is already known on this subject?
Several meta analyses have been conducted with hyaluronic acid that yielded conflicting results.
What does this study add?
This meta analysis was conducted with randomised controlled trials of low risk of bias to avoid any garbage in-garbage out phenomenon.
How might this impact on clinical practice?
This meta analysis supports the use of hyaluronic acid to alleviate pain in patients with knee osteoarthritis.
Introduction
Apart from its large impact on disability,1 knee osteoarthritis (KOA) has recently been found to be associated with increased risk of mortality.2 ,3 The main risk factors for this excess mortality are cardiovascular disease and walking disability,2 ,3 which highlights the importance of alleviating pain and improving function in such patients by promoting a non-sedentary lifestyle.
In clinical practice, both acetaminophen and non-steroidal anti-inflammatory drugs (NSAIDs) are widely used as analgesics for OA. However, patients with KOA often have comorbidities such as obesity and hypertension,4 which precludes the use of such analgesics in all patients. Indeed, in addition to the well-known toxicity of NSAIDs,5 the effect of acetaminophen on OA symptoms are modest at best (effect size 0.18 (0.11–0.25).6 ,7 Also, recent data point to cardiovascular side effects and gastrointestinal toxicity, which raise concerns about the risk/benefit ratio of this widely prescribed drug.8
In addition to oral treatments, intra-articular hyaluronic acid (IAHA) are often used in daily practice for managing KOA. The effectiveness of IAHA, used for more than 20 years, has been much debated. Initially recommended by the Osteoarthritis Research Society International (OARSI),9 the European League Against Rheumatism10 and the American College of Rheumatology (ACR),11 HA is now not recommended by the American Academy of Orthopedic Surgeons12 and conditionally recommended by the ACR;13 the OARSI recently provided an uncertain recommendation.6
Expert opinion from these international societies was mainly based on results from meta-analyses (MAs), which are essential for evidence-based decision-making in clinical practice. Two of the last MAs of HA14 ,15 highlighted some pitfalls in the methodological quality of some randomised controlled trials (RCTs) of HA. This point is crucial because metaepidemiological studies have well demonstrated that the poor methodological quality of trials can be associated with a biased estimation of the treatment effect, which in turn can lead to biased estimations from the MA (‘garbage in—garbage out’).16
To clarify the debate on the efficacy of HA, we performed a new MA restricted to trials of IAHA with the lowest risk of bias. Indeed, such an MA is considered to provide the highest level of evidence available for evaluating an intervention.
Methods
This study is reported according to the preferred reporting items for systematic reviews and MAs (PRISMA) checklist.17 It was performed according to a protocol established before the start of the literature search and data analysis.
Eligibility criteria
We included trials that met the following criteria: (1) RCT, (2) unconfounded comparison of IAHA to IA placebo injection (saline solution) in patients with KOA, and (3) highest methodological quality defined by adequate randomisation and concealment of randomisation and double-blind design. The criteria of low risk of bias were chosen from results of an explanatory analysis of subgroups by Rutjes et al15 and previous meta-epidemiological studies.18 ,19
Information source and search
We systematically searched MEDLINE via PubMed, EMBASE via OVID, and the Cochrane Central Register of Controlled Trials (CENTRAL), the last search performed in December 2013, for published original articles without any restriction on language. We used a search strategy of free text terms and MeSH terms relevant to HA, viscosupplementation and KOA. In a first step, we searched for the following terms: “Hyaluronic Acid” [MeSH Terms]+OR+“Viscosupplementation” [MeSH Terms]+OR+“Viscosupplements” [MeSH Terms])+AND+(“Osteoarthritis, Knee” [MeSH Terms])+AND+(Randomized Controlled Trial [pt]). In a second step, we searched for the subsequent terms: Hyaluronic Acid [MeSH Terms] OR Hyaluronic Acid [TIAB] OR hyaluronate [TIAB] OR Hyaluronan [TIAB] OR hyaluron [TIAB] OR hylan [TIAB] OR synvisc [TIAB] OR orthovisc [TIAB] OR ostenil [TIAB] OR suplasyn [TIAB] OR arthrum [TIAB] OR synovial [TIAB] OR artz [TIAB] OR biotty [TIAB] OR go-on [TIAB] OR healon [TIAB] OR hyaject [TIAB] OR hyalgan [TIAB] OR hyalart [TIAB] OR hyalectin [TIAB] OR nuflexxa [TIAB] OR euflexxa [TIAB] OR polireumin [TIAB] OR hygag [TIAB] OR nrd101 [TIAB] OR replasyn [TIAB] OR supartz [TIAB] OR artzal [TIAB] OR supartz [TIAB] OR Viscosupplementation [MeSH Terms] OR Viscosupplementation [TIAB] OR Viscosupplementations [TIAB] OR Viscosupplements [MeSH Terms] OR Viscosupplements [TIAB] AND Osteoarthritis, Knee [MeSH Terms] OR Knee Osteoarthritides [TIAB] OR Knee Osteoarthritis [TIAB] OR Osteoarthritis Of Knee [TIAB] OR Osteoarthritis Of Knees [TIAB]) AND (“clinical"[TIAB] AND “trial"[TIAB]) OR “clinical trials” [MeSH Terms] OR “clinical trial” [Publication Type] OR “random” [TIAB] OR “random allocation” [MeSH Terms] OR “therapeutic use” [MeSH Subheading]). We also searched the reference lists of all identified relevant studies and review articles, conference abstracts, books and the ClinicalTrials.gov registry for eligible articles.
Study selection
We screened titles and abstracts of identified articles and obtained the full-text article for potential reports for further assessment. Studies that did not meet all criteria were excluded.
Data collection process
Data extraction involved a computer-assisted standardised data-collection form with detailed instructions for data extraction and coding. From eligible articles, we collected data on study characteristics (sample size, number of treatment groups, study design, follow-up duration, funding source, registry number); patient characteristics (sex, age, duration of symptoms, disease severity); interventions (type of comparator, type, dose, intensity, and duration of treatment); outcome measures (mean (SD) baseline and final score, analysis of covariance estimates (SE) when available for each group); and information needed to assess the risk of bias and methodological quality.
Summary measures
The prespecified main outcome was pain intensity at a prespecified end point—3 months—or the nearest end point if missing, and the secondary outcome was physical function (Western Ontario and McMaster Universities Arthritis Index or Lequesne index).
Synthesis of results
The impact of viscosupplementation compared with placebo (saline injection) was expressed as the standardised mean difference (SMD) with its 95% CI by the Hedges’ g estimator. The SMD is the mean divided by the standard deviation (SD) of the difference in pain or function between the viscosupplementation and placebo groups. Data were pooled using a random-effects model and the DerSimonian and Laird method20 to give a more conservative estimate of the effect of viscosupplementation, allowing for any heterogeneity between studies. To better appreciate the clinical relevance of the magnitude of the treatment effect, we used back-transformation of the SMD to an OR.21 ,22 The corresponding OR for the SMD was derived as follows: OR=exp (1.81*d). The OR estimating the relative risk is a more common measure of treatment effects than the SMD. This derived OR extrapolates the value of the OR that would have been obtained if a discretisation of the visual analog scale (VAS) had been performed with a treatment failure threshold. Thus, an OR=0.5 means that the treatment reduces, by dividing by 2, the odds of treatment failure (approximately the risk of treatment failure).
Risk of bias across studies
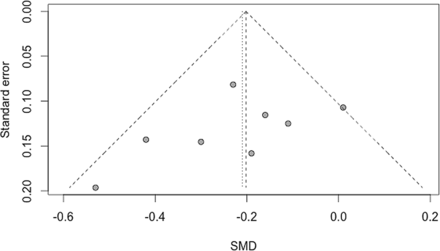
Statistical heterogeneity in study results was explored by a visual inspection of forest plots and quantified by I2 and τ2 statistics.23 Potential publication bias was explored by a visual inspection of funnel plots and Egger's test for funnel asymmetry.24
All analyses involved the use of R and the Meta package (R Core Team 2014).
Results
Study selection
Our literature search yielded 445 potentially relevant articles. We added 147 references, and after excluding duplicates, reviews and non-eligible studies by reading the titles and abstracts, we retrieved 141 potential full-text papers. Finally, we included eight articles of trials of IAHA versus placebo (figure 1).25–32 Table 1 shows the characteristics of the included reports.
Characteristics of the studies included in the meta-analysis of intra-articular injection of HA versus placebo (saline solution)
Flow of the search for articles and trial assessment.
Trial characteristics
Articles for the eight trials were published between 1983 and 2011 and randomised 2199 patients. All trials used a parallel-group design and all had an adequately generated random sequence, adequately concealed treatment allocation and adequately blinded patients and outcome assessors. The length of the studies ranged from 12 to 160 weeks and sample sizes from 168 to 588 (table 2).
Characteristics of patients included in the meta-analysis
Effect on joint pain
The eight trials provided data on pain level (VAS) at month 3. The SMD (random-effects model) was −0.21 (95% CI −0.32 to −0.10) favouring IAHA (figure 2), which corresponds to an OR of 0.68. We detected no significant heterogeneity between trials: I2=32% and τ2=0.007.
Forest plot of differences in pain intensity expressed as effect size (standardised mean difference) at 12 weeks (8 trials).
Effect on joint function
Five trials contributed to the MA of function-related outcomes. The SMD was −0.12 (95% CI −0.22 to −0.02), which corresponds to an OR of 0.80; we found no heterogeneity among trials (I2=0% and τ2=0.0) (figure 3).
Forest plot of differences in function expressed as effect size (standardised mean difference) at 12 weeks (5 trials).
Discussion
The findings from our systematic review and MA restricted to high-quality trials of IAHA versus placebo additionally contribute to the debate on the efficacy of IAHA for KOA. We found an effect size (SMD) for pain of 0.21, which is moderate but clinically relevant on an individual patient basis according to the Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials (IMMPACT) consensus.33
Several reviews and MAs examining the effects of IAHA have produced divergent results.14 ,15 ,34–40 Most MAs (n=7) found HA to be more effective than placebo or marginally effective, and only two studies found that HA did not differ from placebo.38 ,39
One explanation for this discrepancy could be differences in the trials pooled and the data extracted for the MA. From the 89 clinical trials potentially eligible for our review, only eight were at low risk of bias and thus included in our study. Most of our RCTs of IAHA did not fulfil the criteria for a high-quality trial, as was previously found.14 ,15 Pitfalls were mainly related to inadequate randomisation, inadequate concealment and inadequate blinding of patients, which are frequent in trials assessing non-pharmacological treatments of OA.41 ,42
Since low-quality studies might bias the MA itself, we conducted an MA of high-quality trials and prespecified criteria. We chose these criteria according to an explanatory analysis of subgroups15 and results of meta-epidemiological studies:18 ,19 adequate random sequence, concealment of treatment and blinding of patients and outcome assessment.
In directly comparing results from our eight trials,25–32 the SMD for pain at 3 months was −0.20 (95% CI −0.12 to −0.29; fixed-effects model) and −0.21 (−0.10 to −0.32; random-effects model), with I²=32%, τ2=0.0072. These estimates for pain are lower than that reported by Rutjes et al15 (effect size (ES)=0.37 (95% CI 0.28 to 0.46; τ=0.09; Egger test <0.001), who analysed 71 trials. In contrast, our results are close to those reported by Bannuru et al14 (ES for pain −0.29, −0.22 and 0.20 at 12, 16 and 24 weeks, respectively), who restricted their analysis to high-quality trials defined as those with more than 100 randomised participants and reporting intention-to-treat analyses, adequate blinding and allocation concealment.
A global analysis of previously published MAs34–40 showed an ES for IAHA for pain with KOA at 3 months between 0.20 and 0.30. Of note, these estimates are greater than that for acetaminophen (ES=−0.13, 95% CI −0.22 to −0.04),7 a drug recommended by the OARSI6 and the ACR.13 A recent MA comparing the relative efficacy of IAHA and NSAIDs in KOA concluded equal effectiveness of both treatments in alleviating pain at 4 and 12 weeks.43
Given the chronicity of pain in OA and the common presence of cardiovascular comorbidities, the benefits and risks of drugs given for this condition must be carefully weighed. Rutjes et al15 found that HA can increase the risk of serious adverse events. However, these findings, never reported in an MA, have raised severe criticism in terms of the methodology used to assess HA safety.44 ,45 Therefore, given the limited range of available treatments for KOA and the frequency of comorbidities, IAHA may be an appropriate therapeutic option for patients who fail to respond to oral treatments.
The limitations to our MA include the potential for publication bias. Indeed, we included only published trials and therefore cannot exclude that inclusion of some small, negative, non-published studies might have biased our results.46 ,47 In addition, it should be noted that our time point assessment was 3 months, which can be considered a short period of time. In addition, this MA assessed the efficacy of HA as a whole, and did not distinguish separately the different type of HA.
In conclusion, this MA of RCTs of IAHA versus placebo for KOA, with a low risk of bias, shows that IAHA may have a moderate but real benefit for patients with KOA. Given the paucity of well-tolerated effective treatments and the well-known toxicity of NSAIDs, IAHA should be considered to alleviate pain and improve function, in particular for patients with comorbidities.
Acknowledgments
The authors thank Laura Smales for editing the manuscript.
References
Footnotes
Contributors PR, MM and MC conceived the study and interpreted the data. The manuscript was written by PR and MM. XC, HKE, FE, YH, PO and JS revised it critically and provided substantial changes. MC and MM performed the statistical analysis.
Funding This study was funded by Affinité Santé (CRO). The funder was not involved in the design, implementation, results and writing of this study.
Competing interests PR received fees from BioIbérica, Fidia, IBSA, Expanscience, Genévrier, Sanofi, Rottapharm, Servier, Flexion Therapics and Ménarini. XC received fees from Flexion therapics, Moebius, Sanofi and Genévrier. . YH is the founder and chairman of Synolyne Pharma. He received speaker fees from IBSA, BioIberica, Tilman SA and Expanscience. PO received fees from Génévrier and Rottapharm. JS received fees from Expanscience, TRB Chemedica and Servier. MC received fees from Affinités Santés. MM received fees from Pierre Fabre, ChromaPharma, TRB Chemedica, Genévrier, Rottapharm, Daiichi-Sankyo, Ferring, Bioventus and Sanofi.
Provenance and peer review Not commissioned; externally peer reviewed.
Data sharing statement No additional data are available.