Article Text
Abstract
It is evident that the morning symptoms of rheumatoid arthritis (RA) are linked to the circadian abnormal increase in night inflammation, favoured by inadequate cortisol secretion under conditions of active disease. Therefore, exogenous glucocorticoid treatment is recommended in RA at low doses since it may partially act like a ‘replacement therapy’. The prevention/treatment of the night upregulation of the immune/inflammatory reaction (and related flare of cytokine synthesis) has been shown to be more effective when exogenous glucocorticoid administration is obtained with a night-time-release formulation. Large-scale trials documented that modified-release prednisone has greater efficacy then morning prednisone for long-term low-dose glucocorticoid treatment in patients with RA, showing at least a more significant reduction in morning joint stiffness. Interestingly, despite a considerably higher cost than conventional prednisone, chronotherapy with night-time-release prednisone was recognised as a cost-effective option for patients with RA not on glucocorticoids who are eligible for therapy with biological disease-modifying antirheumatic drugs (DMARDs). Moreover, since different cell populations involved in the inflammatory process are particularly activated during the night, other therapeutical approaches used in RA, for example, conventional DMARDs and non-steroidal anti-inflammatory drugs (NSAIDs), should follow the same concepts of glucocorticoid chronotherapy. Indeed, bedtime methotrexate chronotherapy was found to improve RA symptoms compared to the current standard dosing methods, and several available NSAIDs (ie, indomethacin, aceclofenac, ketoprofen, flurbiporfen, lornoxicam) have been very recently modified in their formulation, in order to obtain chronotherapeutical effects in RA.
- Rheumatoid Arthritis
- DMARDs (synthetic)
- NSAIDs
- Inflammation
- Corticosteroids
This is an Open Access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Statistics from Altmetric.com
Key messages
What is already known about this subject?
Morning clinical symptoms of rheumatoid arthritis are linked to circadian abnormal increase of night inflammation, favoured by inadequate cortisol secretion.
What does this study add?
Several evidences now seem to confirm that the treatment of the night up-regulation of the immune/inflammatory reaction at lest in rheumatoid arthritis, is more effective when exogenous glucocorticoid administration is obtained with nighttime-release chronotherapy.
How might this impact on clinical practice?
Treatment of rheumatoid arthritis is starting to include the concept of chronotherapy also for the use of conventional DMARDs and NSAIDs.
Introduction
It is well assessed that crucial clinical signs and symptoms of rheumatoid arthritis (RA) vary within a day and between days, and the morning joint stiffness observed in almost all patients with active RA is also considered one of the most peculiar diagnostic criteria of the disease.1
Similarly, other RA symptoms, such as joint pain and functional disability, are commonly most severe in the early morning by following 24 h cycles, and are a consequence of altered neuroendocrine and immune/inflammatory activities.2
Therefore, it is not surprising that also in other chronic inflammatory rheumatic diseases, including polymyalgia rheumatica (PMR) and ankylosing spondylitis, symptoms of pain and stiffness typically are most prominent during the early morning, similar to RA.3
It is now evident that the morning symptoms, at least in RA, are linked to the circadian increase in proinflammatory cytokines, as a result of an increased night inflammation. Indeed, cytokines, such as tumour necrosis factor (TNF) α and interleukin (IL) 6, are highly increased in patients with active RA in the very late night hours, whereas they are present at very low levels after noon.4
Following several signallings, it is now evident that neuroendocrine circadian rhythms play an important role in RA clinical symptomatology.5 ,6
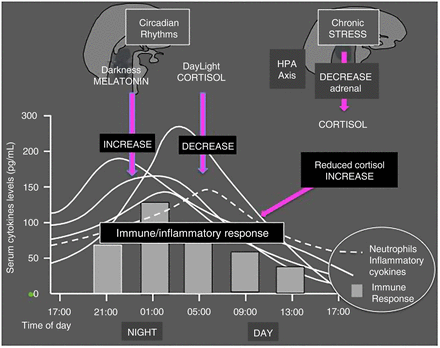
Proinflammatory night hormones, such as melatonin (and prolactin), which follow a 24 h daily cycle, as well as the full availability of bioenergies during the night, are recognised among the triggers/enhancers for increased release and serum concentrations of cytokines7 ,8 (figure 1).
Circadian sequence of nocturnal hormone secretion that induces activation (melatonin, prolactin) and/or dowregulation (cortisol) of the immune inflammatory response during the night. Clinical consequences of altered hormonal balance in rheumatoid arthritis (RA) include morning symptoms such as joint stiffness and pain.
Interestingly, the nocturnal ability of the neuroendocrine system to mount an efficient inflammatory response with related clinical effects also seems to be involved in acute arthritis events such as gout attacks.
The circadian clock and RA
In all individuals, a circadian clock drives daily rhythms in physiology necessary to synchronise the human functions with the 24 h environment.9
Therefore, physiological functions under circadian control include the sleep-wake cycle, heart rate, blood pressure, body temperature, as well as endocrine gland regulation (ie, gonadal and adrenal steroidogenesis) and immune response. These daily rhythms are controlled by a central pacemaker, which is found in a hypothalamic region located above the optic chiasm called the suprachiasmatic nucleus (SCN)10 (figure 2).
The daily neuroimmunoendocrine rhythms (gonadal, adrenal, pituitary hormones) are controlled by a central pacemaker, which is found in a hypothalamic region called the suprachiasmatic nucleus (SCN) of the central nervous system (CNS). APC, antigen presenting cell; DHEA, dehydroepiandrosterone; Th, T helper cell; Treg, regulatory T cell.
The SCN collects from the eyes the light inputs via the retinohypothalamic tract. The central pacemaker synchronises additional peripheral oscillators found locally within organs, tissues and cells.10 These peripheral clocks are synchronised by the central clock, but are self-sustaining and can be entrained by external cues such as temperature.
A bidirectional interaction between inflammation and the circadian clock has been shown recently, and disruption of the clock has a significant effect on the performance of the immune system, with a possible impact on the pathogenesis of RA. Conversely, inflammation can directly alter cellular expression of core clock genes.11
Possible causes of disruption of the clock should include jet lag, causing a desynchrony between the internal clock and the environment, and the condition of night shift-workers. Generally, constant shifts in the daily schedule are detrimental to health and have been linked with an increased incidence of a number of chronic diseases such as cardiovascular disease, metabolic syndromes, diabetes, irritable bowel syndrome and even cancer.12
Interestingly, a study in 2010 provided a significant link also between shift work and an increased risk of RA (in women).13
Once again, the endocrine system mediates the dissemination of timing signals from the SCN throughout the body and to the immune system, and two night hormones act as circadian agents—glucocorticoids and melatonin (prolactin). Both are important in regulation of the immune/inflammatory response, and contribute to the pathogenesis of RA14 ,15 (figure 2).
Insufficient endogenous glucocorticoid secretion in RA
Acute proinflammatory events, such as bacterial infections, activate the hypothalamus-pituitary-adrenal (HPA) axis response, leading to high levels of circulating ACTH and cortisol. In particular, daily production of cortisol can increase by a factor of 18 in extreme situations (ie, first days of sepsis).16
Importantly, this strong stimulation only lasts for a short period of time (a few days). Conversely, inflammation-associated downregulation of the HPA axis activity in chronic inflammation, such as in RA, is related both to circulating cytokines that can harm the HPA axis on all levels (hypothalamus, pituitary gland and adrenal gland) and to a consequential partial adrenal insufficiency17 (figure 3).
Basically, melatonin increases and cortisol reduces the immune/inflammatory reaction following a circadian rhythm. Reduced efficiency of the HPA axis activity related to chronic stimulation, such as in rheumatoid arthritis (RA), can induce a partial adrenal insufficiency. The consequence is reduced cortisol availability and reduced downregulation of the night immune/inflammatory response. HPA, hypothalamus-pituitary-adrenal axis.
The proinflammatory cytokines IL-1β and TNF are the main factors which interfere with several steps of steroidogenesis.
Inadequate secretion of endogenous glucocorticoids is also evident by studying circadian rhythms of serum levels of cytokines and steroids. The circadian rhythm of serum cortisol with respect to amplitude and period is similar in healthy controls and in patients with RA with mild to moderate disease. In contrast, serum levels of IL-6 are almost 10 times higher and the circadian rhythm is quite different in controls and in patients with RA. Therefore, despite raised serum concentrations of IL-6, the amplitude of the circadian rhythm of cortisol is not increased as expected, which is indicative of inadequate cortisol secretion under adrenal stress related to persistent active disease18 (figure 3).
Furthermore, it has been demonstrated that the degradation of the bioactive cortisol to the biologically inactive cortisone is increased when studying mixed synovial cells of inflamed tissue. This phenomenon is due to the increased numbers of cells positive for the degrading enzyme 11β-hydroxysteroid dehydrogenase type 2 and to a decreased reactivation of cortisone to cortisol.19
The clinical and biochemical improvement observed after glucocorticoid therapy in patients with RA appeared in a previous study to be attributed to a direct dampening of proinflammatory factors, as well as to the restoration of the steroid milieu.20
In conclusion, since cortisol is the strongest endogenous anti-inflammatory substance, its abnormal downregulation and hyposecretion during the night in chronic diseases may justify the presence of the early morning clinical symptoms in patients with RA, while in contrast the synthesis of melatonin is still high and enhancing the night inflammatory reaction.20
The role of melatonin in RA
The pineal hormone melatonin exerts a variety of effects on the immune system. Generally, melatonin activates immune cells and enhances inflammatory cytokine and nitric oxide production following a circadian rhythm.5 ,14
Previous investigation showed that in patients with RA and in healthy participants, melatonin levels increase progressively from 20:00 to the early morning hours and they reach peak levels at midnight in patients with RA, which was at least 2 h earlier than in healthy controls.14
Subsequently, melatonin concentrations in patients with RA reach a plateau that lasts for 2–3 h; this was not observed in controls. After 2:00, melatonin levels decrease similarly in patients with RA and in healthy participants. The studies confirmed that the nocturnal rhythm of melatonin occurs also in patients with RA, but with an earlier peak and a longer duration in the early morning.5 ,14
As a matter of fact, production of several cytokines such as IFNγ, IL-1, IL-6, TNF-α, IL-2 and IL-12 (Th1 cytokines) reach the peak during the night, at the same time that melatonin serum levels are highest and plasma cortisol is lowest.
Interestingly, melatonin was found detectable in high concentration in synovial fluids from patients with RA, and binding sites for melatonin were present in synovial macrophages.21 In addition, cultured RA synovial macrophages respond to melatonin stimulation with an increased proinflammatory cytokine production.22 Therefore, it is not surprising that melatonin treatment does not improve RA clinical status, but, to the contrary, may further enhance the inflammatory reaction as previously shown.23
Chronotherapy with low-dose glucocorticoids in RA
Exogenous glucocorticoid treatment is today recommended at low doses in RA since it may act as a ‘replacement therapy’ in the presence of decreased endogenous cortisol availability.24 ,25 However, different mineralocorticoid and glucocorticoid activities are still important aspects that differentiate between exogenous (ie, therapeutic) and endogenous (ie, physiological) glucocorticoids.24 Therefore, exogenous synthetic glucocorticoids exhibit a more selective glucocorticoid/anti-inflammatory action (less mineralocorticoid effects), as well as have a different biological half-life, plasma kinetics, metabolism and non-genomic high-dose effects compared to cortisol (hydrocortisone).
In any case, long-term exogenous glucocorticoid administration may interfere with the HPA axis function and with the circadian cortisol production.
Interestingly, a reduction of mean initial low-dose from 10.3 to 3.6 mg/day (prednisone) on long-term glucocorticoid therapy in RA has been observed in one recent analysis during the period 1980–2004.26
The more specific items of the EULAR recommendations for the management of RA relate to starting disease-modifying antirheumatic drug (DMARD) therapy in early disease using a conventional DMARD strategy in combination with low-dose glucocorticoids.27
In addition, there is evidence that glucocorticoid therapy, especially long-term low-dose treatment, may slow radiographic progression by at least 50% when given to patients with early RA, in agreement with the conventional definition of DMARD.28
Glucocorticoids exert important genomic effects on cellular immunity and, given the existence of cellular circadian rhythms, therefore the prevention of the night upregulation of immune cell activity (and related flare of cytokine synthesis) with their exogenous administration between 6:00 and 8:00 may not be optimal since it is too late to interfere with the activation of the nocturnal inflammatory process.29
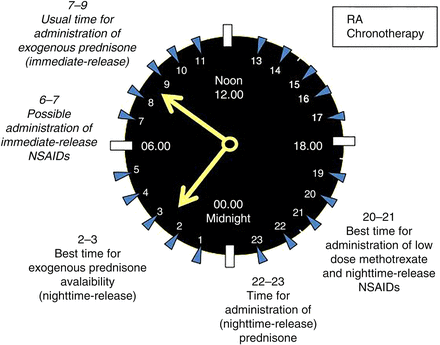
Since it has been established that pain, stiffness and functional disability show maximum levels in the early morning hours, it is now clear that preventing the nocturnal rise of proinflammatory cytokines by glucocorticoids is more effective than treating established symptoms in the morning30 (figure 4).
Low-dose glucocorticoid chronotherapy in rheumatoid arthritis (RA) include the night-time-release prednisone, a timing drug release formulation with administration around 22–23 (22:00–23:00) and releasing prednisone around 2:00–3:00. Other therapeutical approaches used in RA, for example, with disease-modifying antirheumatic drugs (DMARDs: ie, methotrexate) and non-steroidal anti-inflammatory drugs (NSAIDs), should follow the same concept of glucocorticoid chronotherapy.
In addition, several inflammatory pathways also involving the central nervous system involvement in RA (ie, pain perception) might be better controlled by chronotherapy, resulting in increased sleep quality and reduction of related depressive symptoms.6
The first reliable clinical study showing the superiority of night versus morning administration of glucocorticoids in RA was published in 1964.31
Fifty-six patients with RA were included in a double-blind trial. None of the patients had at any time taken a dose of glucocorticoids larger than 5 mg prednisolone. In this trial, patients took one tablet at 22:00 and a second, identical in appearance, in the morning; one tablet contained 5 mg prednisolone and the other was a placebo. For each patient, the 4-week trial was divided into 2-week periods. As result, the 5 mg prednisolone given at night was found to reduce or eliminate morning stiffness in the majority of patients with RA. The effect was quickly apparent and was confirmed by altering the time of administration from night to morning and vice versa in random fashion and under double-blind conditions.31
Twenty years later, in 1984, 41 patients with RA maintained on low-dose prednisolone (mean 5.8 mg) participated in a double-blind crossover study to again determine the effect of timing (morning or night) of prednisolone dosage on morning stiffness.32
The patients were asked to take their study tablets (70%= or <5 mg/day) on retiring (22:00–23:00) and on rising (6:00—7:00) with milk but not a major meal. Prednisolone given at night resulted in a significantly shorter duration of morning stiffness (p=0.0001) than did an equivalent dose given in the morning. In addition, non-parametric statistical analysis (sign test) showed a significant preference (p<0.05) for the night therapy.
Finally, in 1997, 26 glucocorticoid-untreated patients with RA were randomly divided into two equal groups and allocated to low doses of prednisolone at either 2.00 or 7.30.33
Administration of low doses of prednisolone (5 or 7.5 mg daily) at 2:00 after only 5 days showed favourable effects on the duration of morning stiffness (p<0.001), joint pain (p<0.001), Lansbury index (p<0.001), Ritchie index (p<0.001) and morning serum concentrations of IL-6 (p<0.01). The other study group showed minor but significant effects on morning stiffness (p<0.05) and circulating concentrations of IL-6 (p<0.05). The study confirmed that administration of low doses of glucocorticoids with a rather short biological half-life seems to improve acute RA symptoms if it precedes the period of circadian flare in inflammatory activity, as defined by enhanced IL-6 synthesis.
More recently, the most advanced approach for the low-dose prednisone chronotherapy in RA included the modified-release prednisone, a timing drug release with administration at 22:00, and releasing prednisone around 2:00–3:0034 (figure 4). The mean relative change in duration of morning joint stiffness from baseline to end of this study was significantly higher with modified-release prednisone than with immediate-release prednisone (−22.7% vs −0.4%; difference=22.4% (95% CI 0.49 to 44.30); p=0.045). The absolute difference between the treatment groups was 29.2 min (95% CI −2.59 to 61.9) in favour of modified-release prednisone (p=0.072). Finally, in the prednisone modified-release group, a mean reduction of 44.0 (SD 136.6) minutes was achieved compared with baseline.
However, in this study (Circadian Administration of Prednisone in Rheumatoid Arthritis; CAPRA-1), patients with RA treated in the ‘control’ arm were given their daily dose of prednisone early in the morning, and not at bedtime, whereas it should have been administered similarly in the late evening in order to demonstrate the superiority of modified-release prednisone over regular prednisone to reduce morning stiffness.
In addition, no studies compared modified-release prednisone with the standard prednisone but at dosage devided between morning and bedtime.
Treatment with evening modified-release prednisone for up to 12 months was shown to be generally well tolerated, with an overall similar safety profile compared with evening placebo or morning regular prednisone.34 As expected, the incidence of adverse events was higher over the 12-month period than over the 3-month period, even if the increase was not proportional to the duration of treatment.
As a matter of fact, the incidence of severe adverse events during the first 3 months of treatment was 2.4% compared with only 3.3% in patients receiving 12 months of modified-release prednisone administration.34
Large-scale trials documented that modified-release prednisone has greater efficacy for long-term low-dose glucocorticoid treatment in patients with RA, showing a significant reduction in morning joint stiffness/fatigue in addition to all known therapeutic effects with conventional prednisone and a similar safety profile but without additional suppression of the HPA axis.35 ,36
The reported studies confirmed that the specific timing of exogenous prednisone availability, linked to the interaction between IL-6 and the HPA axis, may correct a postulated deficiency in HPA control in RA.37
Effects of the glucocorticoid chronotherapy in RA
Single-centre crossover studies were conducted recently in patients with RA in order to compare the pharmacokinetics of a single 5 mg oral dose of modified-release prednisone and conventional prednisone, as well as the effect of food, on bioavailability.38
No substantial difference in pharmacokinetic parameters of the formulations, apart from the programmed delay in release of prednisone from the night-time-release formulation, was observed. In addition, administration after a full or light meal did not affect pharmacokinetic characteristics; in contrast, the bioavailability was reduced under fasted conditions. Pharmacokinetic evaluation in patients with RA confirmed that modified-release prednisone tablets taken at bedtime with or after an evening meal result in programmed release from 4 to 6 h after intake.38
The effects of long-term low-dose chronotherapy with modified-release prednisone on the HPA axis have been carefully investigated in patients with RA.39 The increase of cortisol plasma concentrations after injection of corticotropin-releasing hormone (CRH) was 5.5 (SD 4.37) μg/dL on regular-morning prednisone at baseline and 5.3 (4.07) μg/dL on modified-release prednisone at 12 months.
Switching from morning to night-time-release prednisone did not influence adrenocortical function, and nor did long-term treatment of up to 12 months with modified-release prednisone. Finally, no worsening of adrenal impairment was observed on treatment with night-time-release prednisone in patients with low responsiveness to CRH testing before treatment with such an approach.
A further observational study assessed functional ability in patients with RA treated with modified-release prednisone under conditions of normal clinical practice.40 Interestingly, the dose of prednisone was significantly reduced after 9 months of treatment (from 5.0 to 4.4 mg/day, p<0.001) with modified-release prednisone and fewer patients with RA required biological DMARD treatments. The advantages related to lowering the mean dose of modified-release prednisone during long-term treatment could include a better safety profile by reducing the risk of adverse effects.
To assess if modified-release prednisone taken at bedtime is confirmed to be more effective than prednisone taken in the morning, a total number of 950 outpatients with RA treated with different morning glucocorticoids and DMARDs (15.8% biologics, 83.7% methotrexate, 10.5% leflunomide) were switched from immediate-release prednisone or 6-methyl (6M)-prednisolone to low-dose modified-release prednisone and followed for 4 months.41
In detail, 513 patients with RA were switched to modified-release prednisone from immediate-release prednisone (9.4±5.4 mg) and 437 from 6M-prednisolone (6.7±3.7 mg). At the time patients with RA completed 4 months (904, 96.8%) of modified-release prednisone treatment after switch from immediate-release prednisone or 6M-prednisolone, morning stiffness duration, pain intensity, patient and physician global assessment improved significantly (p<0.001) when compared to basal time. Moreover, mean daily modified-release prednisone dosage also decreased from 8.2 to 6.7 mg between baseline and end point (16 weeks).
Generally, glucocorticoid-naive patients with RA seem to be the best responders to the night-time-release prednisone. In addition, switching from other glucocorticoids to low-dose modified-release prednisone improved significantly the disease outcomes over 4 months, as shown in the open, unblinded study. 41
Interestingly, in a very recent study assessing the efficacy and safety of night-time-release prednisone versus morning-release prednisone in newly diagnosed patients with PMR, there was a clear consistent trend for a significantly stronger effect of modified-release prednisone across most secondary efficacy end points at week 4, with a discernible treatment difference observed as early as week 1.42
In addition, night-time-release prednisone reduced IL-6 levels in a more significant manner versus morning-release prednisone (p<0.01).
After safety and effectiveness of chronotherapy was assessed, it was evaluated in patients with RA the cost-effectiveness of modified-release formulations compared with immediate-release prednisone and based on duration of morning stiffness.43
Health benefits were evaluated as quality-adjusted life years and the final output was the incremental cost-effectiveness ratio. Efficacy data were derived from the original study on a modified-release prednisone study, drug costs from the British National Formulary.
As expected, the mean treatment costs per patient were found to be higher for night-time-release prednisone than for immediate-release prednisone. The analysis pointed out that the mean treatment costs per patient per year were higher for modified-release prednisone (£649.70) than for regular prednisone (£46.54).
However, the model generated an incremental QALY of 0.044 in favour of modified-release prednisone, which resulted in an ICER of £13 577.
Probabilistic sensitivity analysis reported that modified-release prednisone had an 84% probability of being cost-effective at a willingness-to-pay threshold of £30 000 per QALY.
In synthesis, this analysis demonstrates that, on the basis of the CAPRA-1 trial, modified-release prednisone is a cost-effective treatment option when compared with immediate-release prednisone for patients with RA with morning stiffness over 1 year.
A further study on cost-effectiveness analysed the effects of a 12-week treatment with modified-release prednisone versus placebo on the costs of drug treatment of RA.44
The results showed 11–13% more patients on modified-release prednisone than on placebo treatment reached significant improvement and dropped below reimbursement thresholds of disease activity (DAS28) in the countries of the Netherlands, Belgium and the UK. Interestingly, assuming 1 year of biologics cost €15 000 and MR-pred costs €1/day, €396 are saved in each patient with RA by delaying biological treatment by 12 weeks.
The study again concluded that despite a considerably higher cost than conventional prednisone, chronotherapy with night-time-release prednisone is a cost-effective option for patients with RA not on glucocorticoids who are eligible for therapy with biological DMARDs.44
Other approaches to chronotherapy in RA
A recent study showed that in RA some immune cell populations (ie, monocytes) lose their normal circadian rhythms, and others establish new ‘inflammatory’ circadian rhythms.45
Therefore, since different cells involved in the inflammatory process are particularly activated during the night, other therapeutical approaches used in RA, for example, with DMARDs and non-steroidal anti-inflammatory drugs (NSAIDs), should follow the same concepts of glucocorticoid chronotherapy.
As a matter of fact, the circadian activation of the cells involved in the RA immune/inflammatory response represents the preferential target for conventional and biologic DMARDs, therefore, also the administration of antiproliferative drugs (ie, methotrexate, leflunomide, cyclophosphamide, etc.) should consider those rhythms.
This hypothesis was tested in an in vivo investigation using an animal model of arthritis, and showed that the optimal dosing time, associated with the 24 h cycling of TNF-α, could result in the most efficient methotrexate antiinflammatory activity and the most effective decrease of TNF-α.46
A successive clinical study has confirmed that bedtime methotrexate chronotherapy (3 times a week once a day in the evening) can improve RA symptoms compared to the current standard dosing methods47 (figure 4).
On the other hand, the migration/activation of neutrophilic polymorphonuclear cells in the inflammatory site follows night rhythms, and starting early in the 1980s, various controlled-release NSAIDs have been explored for administration-time differences in their symptomatic effects48 (figure 4).
An early double-blind crossover study design that included multiple (4 to 6 times per day) pain, stiffness and hand strength self-assessments in patients with RA reported that a twice-daily flurbiprofen schedule that lacked an evening dosing time (ie, 200 mg in the morning and 200 mg at midday) was less effective in modulating morning RA signs and symptoms than ones that did (200 mg in the morning and 200 mg at bedtime or 200 mg at midday and 200 mg at bedtime).49
A similar study design found that an evening once-daily scheduling of 75 mg (indomethacin formulation 25 mg immediate-release combined with 50 mg controlled-release) resulted in much greater control of morning OA symptoms compared to once-daily morning (breakfast time) or once-daily midday (lunchtime) schedules.50
Recently, a pH-responsive dual pulse multiparticulate dosage form containing ketoprofen was tested in RA and was found to be able to relieve circadian symptoms during midnight and early morning.51
More recently, with the main intent of delivering maximum concentration of indomethacin available from the dosage form, an oral compression-coated tablet was developed with a predetermined lag time of 6 h before immediate release and which is suitable for treating night RA circadian inflammation.52
Similar results should be obtainable in chronopharmacological treatment of morning RA symptoms with recently synthesised eudragit-coated aceclofenac-loaded pectin microspheres or lastly with a pH-triggered delayed-release colon-specific aceclofenac microspheres.53 ,54
Very recently, a formulation of mini-tablets-filled-pulsincap delivering lornoxicam for chronotherapy of RA was synthesised for night administration.55 The optimised pulsincap formulation releases lornoxicam after a lag time of 5 h and a maximum portion of the drug will be released in the early morning hours.
In conclusion, the ongoing research on biological rhythms in inflammation and the synthesis of circadian agents is leading to a better understanding of the mechanisms of inflammation and to a more rational use of the drugs in patients with RA.
Conclusions
In patients with RA, the stiffness and functional joint disability characterising the early morning hours are related to the night activation of the immune/inflammatory response.
The prevention/treatment of the upregulation of immune cell activity (and related flare of cytokine synthesis) has been shown to be more effective when exogenous glucocorticoid availability is obtained at night-time. The positive results obtained in RA with modified-release prednisone low-dose chronotherapy, following the chronobiology of the disease, seem applicable in RA even for other agents such as conventional DMARDs and NSAIDs.
References
Footnotes
Contributors Research funds to the University of Genova—Division of Rheumatology from Horizon, Mundipharm, Actelion and BMS.
Competing interests None declared.
Provenance and peer review Commissioned; externally peer reviewed.
Data sharing statement No additional data are available.