Article Text
Abstract
Objectives Differentiating IgG4-related disease (IgG4-RD) from multicentric Castleman’s disease (MCD) is challenging because both diseases present high serum IgG4. The objective of this study is to clarify the differences in characteristics and identify a clinically useful approach to differentiate these two diseases.
Methods Forty-five consecutive patients with untreated active IgG4-RD and 33 patients with MCD were included in this study, who visited our institution from January 2000 to August 2016. The clinical and laboratory findings for the patients of the two diseases were compared. Various combinations of the distinctive findings were evaluated to identify the most efficient differentiating features between IgG4-RD and MCD.
Results The levels of serum IgG4 were not different between the two diseases. Orbits, lacrimal glands, salivary glands or pancreas were involved in 88.9% of IgG4-RD cases and only in 3.0% of MCD cases. All MCD cases involved lymph nodes. Atopic history was characteristic for IgG4-RD. The levels of C reactive protein (CRP) with a cut-off of 0.80 mg/dL and IgA with a cut-off of 330 mg/dL were the most distinctive. The combination of ‘Orbits, lacrimal glands, salivary glands or pancreas involvement, atopic history, or non-involvement of lymph node’ and ‘CRP ≤ 0.8 mg/dL or IgA ≤ 330 mg/dL’ yielded the probability of 97.8% in IgG4-RD, while that of 3.0 % in patients with MCD.
Conclusions Our study revealed distinct features between IgG4-RD and MCD. Differentiating between the diseases based on those distinct features, including distribution of organ involvement, atopic history, levels of IgA and CRP, was a useful approach.
- Autoimmune diseases
- Autoimmunity
- Inflammation
This is an Open Access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Statistics from Altmetric.com
Key messages
What is already known about this subject?
Distinguishing IgG4-related disease (IgG4-RD) from multicentric Castleman’s disease (MCD) is challenging.
What does this study add?
This study has provided a clinically useful approach to distinguish IgG4-RD from MCD based on the distribution of organ involvement, atopic history, levels of IgA and C reactive protein.
Serum IgG4 levels did not distinguish IgG4-RD from MCD.
How might this impact on clinical practice?
Particularly, in difficult cases to distinguish IgG4-RD from MCD, our approach might help to make the diagnosis of IgG4-RD or MCD.
Introduction
IgG4-related disease (IgG4-RD) is an immune-mediated, fibroinflammatory, multisystem disease characterised by tumour-like swelling of involved organs, elevation of serum IgG4 levels and histopathological features of lymphoplasmacytic infiltration rich in IgG4+ plasma cells.1–3 IgG4-RD diagnosis is based on the comprehensive diagnostic criteria proposed in 2011;4 however, many other diseases can present with features of IgG4-RD and differentiating among them could be challenging.5–7
Castleman’s disease (CD), a rare polyclonal lymphoproliferative disorder,8 is sometimes confused with IgG4-RD. Despite the distinct differences in pathogenesis, treatment and prognosis between the two diseases,9–11 multicentric CD (MCD) involving multiple lymphoid lesions and other various organs12 is sometimes accompanied by the elevation of serum IgG4 levels and infiltration of IgG4+ plasma cells in the affected organs, which could fulfil the diagnostic criteria for IgG4-RD.13–16 Therefore, the diagnostic criteria for IgG4-RD and the international consensus guidance statement on the management and treatment of IgG4-RD have recommended careful differentiation from MCD in diagnosing IgG4-RD.4 17 It is known that the levels of serum interleukin (IL) 6, playing a central role in the pathogenesis of MCD, are significantly different between the two diseases;18–20 however, more clinically applicable methods to differentiate between the two diseases are essential in practice.
The aim of this study is to clarify the differences in clinical characteristics, including distribution of organ involvements and laboratory findings between patients with IgG4-RD and MCD, and to propose an approach of distinguishing between the two diseases.
Methods
Patients and clinical data
Written informed consent from patients was waived in accordance with the regulation in Japan. All investigations were conducted according to the principles in the Declaration of Helsinki.
We retrospectively reviewed all Japanese patients with IgG4-RD and CD who visited the rheumatology or haematology department in our institution from January 2000 to August 2016. Patients who had not been treated with any immunosuppressant or glucocorticoids on diagnosis were included, and patients with unicentric Castleman’s disease (UCD), HIV-positive MCD and Castleman-Kojima disease (TAFRO syndrome; Thrombocytepenia, Anasarca, MyeloFibrosis, Renal dysfunction, Organomegaly) were excluded because these diseases, presently, are regarded as different clinical entities from MCD.21–23 IgG4-RD cases, which were diagnosed based on the comprehensive diagnostic criteria4 with pathological confirmation, and MCD cases diagnosed by polylymphadenopathy with the typical histopathological lymphadenopathic lesion such as atrophic germinal centre, mature plasma-cell sheet proliferation, hyaline vascular lesion, enlargement of interfollicular area and haemosiderin deposition, were included in the analysis as ‘cases with pathological diagnosis’ to compare the characteristics between the two diseases. All the diagnoses were confirmed by pathologists in our hospital. Patients with IgG4-RD satisfying comprehensive diagnostic criteria but not having pathological confirmation and patients with MCD pathologically diagnosed by biopsy specimens from other organs than lymph nodes were regarded as ‘cases with clinical diagnosis.’ Clinical information including age, sex, atopic history, distribution of organ involvement and laboratory findings at diagnosis were collected from patients’ medical records. Atopic history was defined as the history of allergic rhinitis, asthma and atopic dermatitis. Organ involvement was assessed by physical examinations and systemic, radiological examinations including CT, MRI and/or fluoro-D-glucose positron emission tomography (FDG-PET). The involvement of lymph nodes was evaluated by whole body CT and/or FDG-PET. Serum IgG4 was measured with Binding Site immunoassay kit (normal range: 4–108 mg/dL).
Significance of each characteristic and their combinations to differentiate between the two diseases
The clinical characteristics of IgG4-RD and MCD cases with pathological diagnosis were statistically compared. We extracted the statistically different data, and classified them into four categories: inflammatory features, allergic features, immunoglobulin patterns and distribution of organ involvements. Next, we assessed each characteristic by sensitivity, specificity and the area under the curve (AUC) of a receiver operating characteristic (ROC) curve for IgG4-RD diagnosis. From each category, one or two characteristics with the highest AUC, sensitivity or specificity were selected and classified as clinical features or immunological features of the disease. These characteristics were combined in various ways, and each combination included at least one clinical and immunological feature. The percentage of patients featuring in these combinations was calculated.
Classification tree for distinguishing IgG4-RD and MCD
We constructed classification tree of the cases with pathological diagnosis in our study described by Bloch et al 24 for distinguishing IgG4-RD from MCD. To validate this classification approach, the same tree analysis was performed using the cases with clinical diagnosis.
Statistical analysis
Continuous variables were presented as mean±SD and categorical variables were presented as numbers and percentages. Statistical differences for continuous variables were assessed by Student’s t-test or the appropriate non-parametrical test, depending on normal or non-normal data distribution. Categorical variables were analysed by Fisher’s exact test. AUC for the diagnosis of IgG4-RD was calculated by the ROC method. The value of p <0.05 (two-sided) was considered as statistically significant. SPSS V.23.0 (IBM, Armonk, New York, USA) was used for all statistical analyses.
Results
Patients’ inclusion
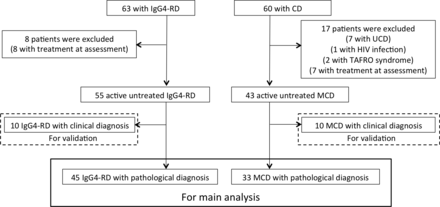
Figure 1 summarises the inclusion and exclusion of patients. In total, 63 consecutive patients with IgG4-RD and 60 patients with CD were identified. Of those, 7 patients with UCD,1 patient infected with HIV, 2 with TAFRO syndrome and 15 (8 with IgG4-RD and 7 with MCD) who had already received immunosuppressive treatment at diagnosis were excluded. We separated them into two parts; 78 patients (45 with IgG4-RD and 33 with MCD) as cases with pathological diagnosis and 20 patients (10 with IgG4-RD and 10 with MCD) as cases with clinical diagnosis. All IgG4-RD cases satisfied the comprehensive diagnostic criteria for IgG4-RD, while six MCD cases with pathological diagnosis and one MCD case with clinical diagnosis satisfied the criteria for IgG4-RD (see online supplementary table 1).
Flow chart of inclusion and exclusion of patients. IgG4-RD, IgG4-related disease; CD, Castleman’s disease; UCD, unicentric Castleman’s disease; MCD, multicentric Castleman’s disease.
Area under the curve (AUC) of the receiver operator characteristic (ROC) curve, sensitivity and specificity of each IgG4-RD characteristic
Patient demographics and laboratory findings at diagnosis
Demographics of cases with pathological diagnosis are shown in figure 2A. Patients with IgG4-RD were older than those with MCD, and atopic history was more frequently observed in patients with IgG4-RD than in those with MCD (71.1% vs 24.2%, p <0.001). While orbits and lacrimal glands (75.5% vs 3.0%, p <0.001), salivary glands (64.4% vs 3.0%, p <0.001) and pancreas (22.2% vs 0%, p <0.001) were more frequently affected in patients with IgG4-RD, lymph nodes (46.6% vs 100%, p <0.001), lungs (26.6% vs 54.4%, p=0.003), skin (4.4% vs 21.2%, p <0.001) and spleen (0% vs 48.5%, p <0.001) were more frequently involved in MCD.
Comparison of patient characteristics between patients with IgG4-RD and those with MCD. (A) Clinical characteristics and distribution of organ involvement. (B) Laboratory data. An asterisk denotes p<0.05. Some data were not measured in all patients, and the list of data and the available number of patients are as follows; a, IgG4-RD n=43; b, IgG4-RD n=43; c, MCD n=6; d, IgG4-RD n=28, MCD n=12; e, IgG4-RD n=35, MCD n=18; f, IgG4-RD n=36, MCD n=18; g, IgG4-RD n=36, MCD n=18; h, IgG4-RD n=39, MCD n=10; i, IgG4-RD n=29, MCD n=17; j IgG4-RD n=36, MCD n=26; k IgG4-RD n=33; l, MCD n=6; m, IgG4-RD n=43; n, IgG4-RD n=44. Alb, albumin; CRP, C reactive protein; ESR, erythrocyte sedimentation rate; Hb, haemoglobin; IgG4-RD, IgG4-related disease; LDH, lactate dehydrogenase; MCD, multicentric Castleman’s disease; Plt, platelet; TP, total protein; TC, total cholesterol; WBC, white blood cell.
Laboratory findings at diagnosis are shown in figure 2B. Patients with MCD had significantly higher levels of IgG (1917.3 ± 759.0 mg/dL vs 4689.5±1868.9 mg/dL, p <0.001), IgA (191.6±81.6 mg/dL vs 667.4±301.9 mg/dL, p <0.001), IgM (86.9±48.5 mg/dL vs 293.7±180.7 mg/dL, p <0.001), while the IgG4 (564.0±451.8 mg/dL vs 505.8±320.1 mg/dL, p=0.943) and IgE (819.6±1044.4 IU/mL vs 1073.4±1017.0 IU/mL, p=0.096) were not statistically different between patients with IgG4-RD and MCD. The proportion of eosinophils in the total leucocyte count was significantly higher in patients with IgG4-RD than in those with MCD (6.4±5.9% vs 2.9%±2.7%, p=0.001). Serum levels of C reactive protein (CRP: 0.3±0.8 mg/dL vs 5.9±4.3 mg/dL, p <0.001) and erythrocyte sedimentation rate (ESR: 33.7±33.0 mm/hr vs 112.8±31.1 mm/hr, p <0.001) were significantly increased in MCD.
Significance of proposed characteristics in differentiating between the two diseases
As shown in table 1, we placed the significantly different findings identified in the previous comparison system in four categories: inflammatory features (CRP, haemoglobin, albumin, total protein, total cholesterol, lactate dehydrogenase, platelet count and soluble IL-2 receptor), allergic features (atopic history and eosinophils), immunoglobulin patterns (IgG, IgA, IgM and IgG4/IgG) and distribution of organ involvement (orbits, lacrimal glands, salivary glands, pancreas, lymph nodes, lungs, skin and splenomegaly). Table 1 shows the sensitivity, specificity and AUC (wherever applicable) of each variable for IgG4-RD. The variables with the highest AUC, sensitivity or specificity in each compartment were: CRP (AUC 0.96 with a cut-off of 0.80 mg/dL, sensitivity 88.8%, specificity 93.9%) in the inflammatory features category; atopic history (sensitivity 71.1%, specificity 75.7%) in the allergic features category; IgA (AUC 0.96 with a cut-off of 330 mg/dL, sensitivity 93.2%, specificity 93.9%) in the immunoglobulin patterns category; and involvement of orbits, lacrimal glands, salivary glands or pancreas (sensitivity 88.9%, specificity 97.0%) and non-involvement of lymph nodes (sensitivity 53.4%, specificity 100%) in the distribution of organ involvement category.
Thereafter, we grouped the three variables—involvement of orbits, lacrimal glands, salivary glands or pancreas, atopic history, and non-involvement of lymph nodes—under clinical features, and the two features—CRP and IgA—under immunological features. In order to calculate the probability of IgG4-RD and MCD, the variables under clinical and immunological features were combined such that each combination included at least one of each feature type (figure 3). The most differentiating combination was ‘orbits, lacrimal glands, salivary glands, or pancreas involvement, atopic history, or non-involvement of lymph node’ and ‘CRP ≤0.8 mg/dL or IgA ≤330 mg/dL’, which coincided with the characteristics of 97.8% of patients with IgG4-RD and only 3.0% of patients with MCD.
Probability of diagnosis of IgG4-RD and MCD for various combinations of clinical and immunological features. The unit of number is per cent. CRP, C reactive protein; IgG4-RD, IgG4-related disease; MCD, multicentric Castleman's disease.
Tree for distinguishing IgG4-RD and MCD
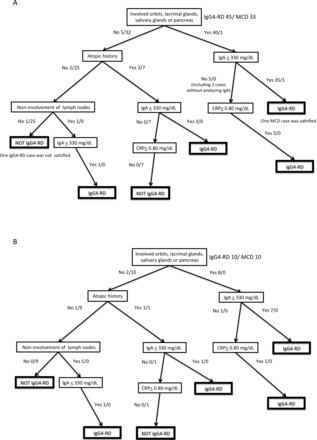
Figure 4A shows the classification tree of the IgG4-RD and MCD cases with pathological diagnoses. Involvement of orbits, lacrimal glands, salivary glands, or pancreas and IgA levels of ≤330 mg/dL could distinguish 35 of 45 patients with IgG4-RD from MCD. Atopic history, CRP levels of ≤0.8 mg/dL or non-lymph node involvement helped distinguish 9 of the remaining 10 cases of IgG4-RD. Final, only one IgG4-RD and one MCD cases were misclassified as the other disease.
Classification tree with the selected characteristics. (A) Cases with pathological diagnosis: 45 patients with IgG4-RD and 33 patients with MCD. (B) Cases with clinical diagnosis: 10 IgG4-RD and 10 MCD cases. The number of patients was shown as IgG4-RD cases/MCD cases. CRP, C reactive protein; IgG4-RD, IgG4-related disease; MCD, multicentric Castleman's disease.
Online%20supplementary table 2 shows patient characteristics of the IgG4-RD and MCD cases with clinical diagnosis. None of the IgG4-RD cases underwent biopsy because of the difficulty in acquiring pathological samples. MCD cases were examined histologically with tissues from skin in six cases, bone marrow in three cases and lung in one case, because involved lymph nodes were difficult to approach. Figure 4B shows the tree analysis of the cases with clinical diagnosis. All IgG4-RD and MCD cases could be differentiated with involvement of orbits, lacrimal glands, salivary glands, or pancreas, atopic history, non-lymph node involvement, and the levels of IgA and CRP, indicating our method could be useful in cases suspected of IgG4-RD and MCD but difficult for obtaining tissue specimens from involved organs.
Discussion
Our study clarified the similarities and differences between IgG4-RD and MCD characteristics demonstrating that the IgG4 levels are not a reliable distinguishing diagnostic feature for the two diseases; however, organ involvement distribution, atopic history and CRP and IgA levels were quite distinctive. The study also demonstrated that differentiation of IgG4-RD and MCD diagnosis based on the combination of those distinctive features was a useful approach.
With growing recognition of IgG4-RD, it has been found that misdiagnosis of IgG4-RD with MCD and vice versa is not so rare,13–16 18–20 especially in cases lacking the unique symptoms of MCD, such as fever. All our MCD cases measuring serum IgG4 showed IgG4 elevation above the cut-off criterion of the comprehensive diagnostic criteria,4 resulting in ‘possible diagnosis’. Moreover, a study with 9 IgG4-RD cases and 28 MCD cases from Japan reported the difficulty of differentiating between the two diseases by using abundant IgG4+ plasma cell invasion in the organ tissues and serum IgG4 levels.20 However, the rarity of both diseases has prevented a solid comparison of the distinctive clinical and laboratory features between the two diseases. In the present study, we compared clinical and laboratory features in 45 IgG4-RD cases and 33 MCD cases, and proposed a distinctive combination of features for the two diseases. While pathological findings are the most important factors in the diagnosis of the diseases, tissue specimens of organs involved in IgG4-RD and MCD are sometimes difficult to acquire. We believe that our approach, composed of past atopic history, distribution of organ involvement and laboratory findings, is helpful in daily clinical practice, and that it can help in establishing classification criteria for the two diseases.
Distinguishing IgG4-RD from MCD is essential because their treatments are entirely different; a moderate dose of glucocorticoids is usually effective for IgG4-RD but not MCD. A multicentre retrospective survey in Japan revealed that the remission rate of patients with IgG4-related pancreatitis, receiving <40 mg/day of glucocorticoids was 98%,9 while a recent systematic literature review reported that only a quarter of patients with MCD responded to glucocorticoids and 91% of those treated with an anti-IL-6 agent achieved complete response.11 Indeed, reviewing the patients in our study, we found that all patients with IgG4-RD but one case which was treated with rituximab achieved remission with glucocorticoid, but most of the patients with MCD failed to respond to glucocorticoid and responded well to anti-IL-6 agent.
The clinical differences demonstrated in this study highlight differences in the underlying pathogenesis of the two diseases. Although the pathogenesis of IgG4-RD is still unclear, allergic mechanisms driven by T helper 2 (Th2) cytokines from follicular Th2 cells25–28 and/or mast cells29 are considered to play a major role. Sato et al 20 reported that the infiltration of eosinophils was more frequently observed in lymph nodes of IgG4-RD compared with that of MCD, and Kamisawa et al 30 reported that patients with IgG4-related pancreatitis frequently had atopic history and elevated circulating eosinophil count. In line with these studies, the comparison of baseline characteristics in our study demonstrated that the proportion of circulating eosinophils and atopic diseases were significantly higher in patients with IgG4-RD than in patients with MCD, supporting the involvement of Th2 cytokines in the pathogenesis of IgG4-RD.
Our study raises another interesting point regarding IgG4-RD. Whereas IgA and CRP, laboratory markers linked to IL-6, were useful in differentiating IgG4-RD from MCD, three IgG4-RD cases showed elevated serum IgA levels with normal CRP, and another three IgG4-RD cases showed normal IgA with elevated CRP. IgA production is promoted by transforming growth factor-β (TGF-β) ,31 which is highly expressed in affected sites of IgG4-RD and clonally expanding CD4+ cytotoxic T lymphocytes.32 33 On the other hand, some entities of IgG4-RD such as IgG4-related retroperitoneal fibrosis and periaortitis frequently present CRP elevation.34–36 Several studies have shown that those phenotypes of IgG4-RD are closely related to inflammatory aortic aneurysm which elevates serum CRP through inflammation in adventitia with massive infiltration of lymphoplasmacytes.37 38 This finding indicates the complexity of pathogenesis of IgG4-RD.
Our study has some limitations. Although we included patients diagnosed with pathological confirmation in the analysis and validated our findings using the cases with clinical diagnosis in the largest cohort of two diseases, our results may be affected by institutional bias because of the nature of a single-centre, retrospective study. The identified characteristics should be validated in a multicentre cohort in the future. Second, our inclusion criteria for MCD in the main analysis were not exactly the same with the diagnostic criteria for MCD proposed quite recently.39 We enrolled only patients with MCD with pathological diagnosis by specimens from lymph nodes, the findings of which were similar with those stated in the diagnostic criteria for MCD. The pathological findings of our cases were confirmed by pathologists in our hospital for the accurate diagnosis.
In conclusion, the distribution of organ involvement was quite different between the two diseases. Moreover, presence of an atopic history was characteristic of IgG4-RD whereas intense inflammation and polyclonal hyperimmunoglobulinaemia were characteristic of MCD. Differentiation based on these distinct features, including distribution of organ involvement, atopic history, levels of IgA and CRP, is a useful clinical approach.
Acknowledgments
The authors thank Professor John H Stone (MassachusettsGeneral Hospital and Harvard Medical School) and Dr Zachary S Wallace(Massachusetts General Hospital) for helpful discussion.
Acknowledgments
The authors thank Professor John H Stone (Massachusetts General Hospital and Harvard Medical School) and Dr Zachary S Wallace (Massachusetts General Hospital) for helpful discussion.
References
Footnotes
Contributors TS and MA participated in the study design, reviewed the patient data, performed the statistical analysis, evaluated the results and drafted the manuscript. YK, TM, HY, KS and KY participated in the study design and helped to draft the manuscript. SO and TT conceived the study, participated in its design and coordination, evaluated the results and helped to draft the manuscript. All authors have read and approved the final version of the manuscript.
Competing interests TS has received consultancies, speaking fees and honoraria from Eisai Co Ltd. MA has received consultancies, speaking fees and honoraria from Cure Grades Co and Eisai Co Ltd. YK has received consultancies, speaking fees and honoraria from AbbVie GK, Astellas Pharma, Bristol–Myers KK, Chugai Pharmaceutical Co Ltd, Eisai Co, Ltd, Janssen Pharmaceutical KK, Kyowa Hakko Kirin Co Ltd, Mitsubishi Tanabe Pharma Co, Pfizer Japan Inc, Santen Pharmaceutical Co, Taisho Toyama Pharma Co and UCB. TM has received consulting fees, speaking fees and honoraria from Astellas Pharma, Chugai Pharmaceutical, Eisai and Janssen Pharmaceutical KK. HY declares no competing interest. KS has received consulting fees, speaking fees and honoraria from Bristol–Myers Squibb Company, Daichi Sankyo Co Ltd, Eisai Co Ltd and Kissei Pharmaceutical Co Ltd. KY has received consultancies, speaking fees and honoraria from AbbVie GK, Acterlion Pharmaceuticals, Astellas Pharma, Bristol–Myers KK, Chugai Pharmaceutical Co Ltd, Eisai Co Ltd, Eli Lilly Japan KK, GlaxoSmithkline, Janssen Pharmaceutical KK, Mitsubishi Tanabe Pharma Co, Nippon Shinyaku Co Ltd, Pfizer Japan Inc and Takeda Pharmaceutical Co Ltd. SO has received consulting fees, speaking fees and honoraria from Astellas Pharma, Bristol-Myers KK, Chugai Pharmaceutical, Eisai, Pfizer Japan and Takeda Pharmaceutical. TT has received consultancies, speaking fees and honoraria from AbbVie GK, Asahikasei Pharma Corp, Astellas Pharma, Bristol–Myers KK, Chugai Pharmaceutical Co Ltd, Eisai Co Ltd, Janssen Pharmaceutical KK, Mitsubishi Tanabe Pharma Co, Pfizer Japan Inc, Takeda Pharmaceutical Co Ltd and UCB.
Ethics approval The ethics committee at Keio University School of Medicine.
Provenance and peer review Not commissioned; externally peer reviewed.
Data sharing statement No additional data are available.