Abstract
Summary
We completed a network meta-analysis of published papers to compare bisphosphonate gastrointestinal safety. We found that zoledronic acid had the highest chance of causing gastrointestinal adverse events. Etidronate had the highest chance of discontinuation due to an adverse event. No difference was found for serious adverse events.
Introduction
Bisphosphonates are first-line treatment for osteoporosis. Gastrointestinal (GI) adverse events (AE) are the primary reason for non-adherence. Little is known about the comparative GI safety of bisphosphonates.
Purpose
Leverage published clinical trial data to examine the comparative GI safety of bisphosphonates.
Methods
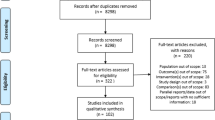
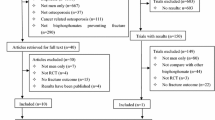
We completed a systematic review of all English-language clinical trials that assessed bisphosphonate safety and/or efficacy in primary osteoporosis through to 2012. Randomized, blinded, and controlled studies were eligible. The primary outcome was any GI-related AE. Subanalyses were completed for upper GI symptoms, serious GI, nausea, esophageal-related events, and discontinuation due to AE. A Bayesian-based network meta-analysis was completed to allow for indirect comparisons. Results were reported as the probability that a specific drug had the highest number of events.
Results
We identified 50 studies: 32 alendronate, 12 risedronate, 5 etidronate, and 7 zoledronic acid. Zoledronic acid had the highest probability of having the highest number of any GI AE (91 %) and nausea (70 %). Etidronate (70 %) and zoledronic acid (28 %) had the highest probability of having the greatest attrition due to AE. Etidronate had the highest probability (56 %) of having the greatest number of upper GI symptoms among oral bisphosphonates.
Conclusion
Zoledronic acid had the highest probability of causing the greatest number of GI AE, possibly related to nausea. These results question the assumption that annual zoledronic acid will translate into better adherence. Little difference was found between alendronate and risedronate for serious AE. More research into real-world implications of the comparative safety of bisphosphonates is needed.



Similar content being viewed by others
References
Adachi JD, Faraawi RY, O’Mahony MF, Nayar A, Massaad R, Evans JK, Yacik C (2009) Upper gastrointestinal tolerability of alendronate sodium monohydrate 10 mg once daily in postmenopausal women: a 12-week, randomized, double-blind, placebo-controlled, exploratory study. Clin Ther 31:1747–1753
Bauer DC, Black D, Ensrud K, Thompson D, Hochberg M, Nevitt M, Musliner T, Freedholm D (2000) Upper gastrointestinal tract safety profile of alendronate: the fracture intervention trial. Arch Intern Med 160:517–525
Black DM, Cummings SR, Karpf DB et al (1996) Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research Group. Lancet 348:1535–1541
Black DM, Delmas PD, Eastell R et al (2007) Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med 356:1809–1822
Blouin J, Dragomir A, Ste-Marie LG, Fernandes JC, Perreault S (2007) Discontinuation of antiresorptive therapies: a comparison between 1998–2001 and 2002–2004 among osteoporotic women. J Clin Endocrinol Metab 92:887–894
Bobba RS, Beattie K, Parkinson B, Kumbhare D, Adachi JD (2006) Tolerability of different dosing regimens of bisphosphonates for the treatment of osteoporosis and malignant bone disease. Drug Saf 29:1133–1152
Boonen S, Orwoll E, Magaziner J et al (2011) Once-yearly zoledronic acid in older men compared with women with recent hip fracture. J Am Geriatr Soc 59:2084–2090
Cadarette SM, Katz JN, Brookhart MA, Sturmer T, Stedman MR, Levin R, Solomon DH (2009) Comparative gastrointestinal safety of weekly oral bisphosphonates. Osteoporos Int 20:1735–1747
Catala-Lopez F, Sanfelix-Gimeno G, Tobias A, Hurtado I, Sanfelix-Genoves J, Peiro S (2013) Efficacy of osteoporosis therapies in a network meta-analysis with indirect comparisons: many concerns for new tools of evidence synthesis? Osteoporos Int 24:1927–1928
Cortet B, Bera-Louville A, Gauthier P, Gauthier A, Marchandise X, Delcambre B (2001) Comparative efficacy and safety study of etidronate and alendronate in postmenopausal osteoporosis effect of adding hormone replacement therapy. Joint Bone Spine 68:410–415
Cramer JA, Amonkar MM, Hebborn A, Altman R (2005) Compliance and persistence with bisphosphonate dosing regimens among women with postmenopausal osteoporosis. Curr Med Res Opin 21:1453–1460
Cranney A, Guyatt G, Griffith L, Wells G, Tugwell P, Rosen C (2002) Meta-analyses of therapies for postmenopausal osteoporosis. IX: summary of meta-analyses of therapies for postmenopausal osteoporosis. Endocr Rev 23:570–578
Curtis JR, Yun H, Matthews R, Saag KG, Delzell E (2012) Adherence with intravenous zoledronate and intravenous ibandronate in the United States Medicare population. Arthritis Care Res 64:1054–1060
Donahue JG, Chan KA, Andrade SE et al (2002) Gastric and duodenal safety of daily alendronate. Arch Intern Med 162:936–942
Eckermann S, Coory M, Willan AR (2009) Indirect comparison: relative risk fallacies and odds solution. J Clin Epidemiol 62:1031–1036
Eisman JA, Rizzoli R, Roman-Ivorra J, Lipschitz S, Verbruggen N, Gaines KA, Melton ME (2004) Upper gastrointestinal and overall tolerability of alendronate once weekly in patients with osteoporosis: results of a randomized, double-blind, placebo-controlled study. Curr Med Res Opin 20:699–705
Freemantle N, Cooper C, Diez-Perez A, Gitlin M, Radcliffe H, Shepherd S, Roux C (2013) Results of indirect and mixed treatment comparison of fracture efficacy for osteoporosis treatments: a meta-analysis. Osteoporos Int 24:209–217
Goodman NF, Cobin RH, Ginzburg SB, Katz IA, Woode DE (2011) American Association of Clinical Endocrinologists Medical Guidelines for Clinical Practice for the diagnosis and treatment of menopause: executive summary of recommendations. Endocr Pract 17:949–954
Hoaglin DC, Hawkins N, Jansen JP et al (2011) Conducting indirect-treatment-comparison and network-meta-analysis studies: report of the ISPOR Task Force on indirect treatment comparisons good research practices: part 2. Value Health 14:429–437
Hopkins RB, Goeree R, Pullenayegum E, Adachi JD, Papaioannou A, Xie F, Thabane L (2011) The relative efficacy of nine osteoporosis medications for reducing the rate of fractures in post-menopausal women. BMC Musculoskelet Disord 12:209
Jansen JP, Bergman GJ, Huels J, Olson M (2011) The efficacy of bisphosphonates in the prevention of vertebral, hip, and nonvertebral-nonhip fractures in osteoporosis: a network meta-analysis. Semin Arthritis Rheum 40(275–284):e271–e272
Jansen JP, Fleurence R, Devine B, Itzler R, Barrett A, Hawkins N, Lee K, Boersma C, Annemans L, Cappelleri JC (2011) Interpreting indirect treatment comparisons and network meta-analysis for health-care decision making: report of the ISPOR Task Force on indirect treatment comparisons good research practices: part 1. Value Health 14:417–428
Kane S, Borisov NN, Brixner D (2004) Pharmacoeconomic evaluation of gastrointestinal tract events during treatment with risedronate or alendronate: a retrospective cohort study. Am J Manag Care 10:S216–S226
Kanis JA, McCloskey EV, Johansson H, Cooper C, Rizzoli R, Reginster JY (2013) European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos Int 24:23–57
Kennel KA, Drake MT (2009) Adverse effects of bisphosphonates: implications for osteoporosis management. Mayo Clin Proc 84:632–637, quiz 638
Kherani RB, Papaioannou A, Adachi JD (2002) Long-term tolerability of the bisphosphonates in postmenopausal osteoporosis: a comparative review. Drug Saf 25:781–790
Lanza FL, Hunt RH, Thomson AB, Provenza JM, Blank MA (2000) Endoscopic comparison of esophageal and gastroduodenal effects of risedronate and alendronate in postmenopausal women. Gastroenterology 119:631–638
Lunn DJ, Thomas A, Best N, Spiegelhalter D (2000) WinBUGS—a Bayesian modelling framework: concepts, structure, and extensibility. Stat Comput 10:325–337
McClung M, Miller P, Recknor C, Mesenbrink P, Bucci-Rechtweg C, Benhamou CL (2009) Zoledronic acid for the prevention of bone loss in postmenopausal women with low bone mass: a randomized controlled trial. Obstet Gynecol 114:999–1007
Miller RG, Bolognese M, Worley K, Solis A, Sheer R (2004) Incidence of gastrointestinal events among bisphosphonate patients in an observational setting. Am J Manag Care 10:S207–S215
Papaioannou A, Morin S, Cheung AM et al (2010) 2010 clinical practice guidelines for the diagnosis and management of osteoporosis in Canada: summary. CMAJ 182:1864–1873
Penning-van Beest FJ, Goettsch WG, Erkens JA, Herings RM (2006) Determinants of persistence with bisphosphonates: a study in women with postmenopausal osteoporosis. Clin Ther 28:236–242
Rosen CJ, Hochberg MC, Bonnick SL et al (2005) Treatment with once-weekly alendronate 70 mg compared with once-weekly risedronate 35 mg in women with postmenopausal osteoporosis: a randomized double-blind study. J Bone Miner Res 20:141–151
Solomon DH, Rekedal L, Cadarette SM (2009) Osteoporosis treatments and adverse events. Curr Opin Rheumatol 21:363–368
Thomson AB, Marshall JK, Hunt RH, Provenza JM, Lanza FL, Royer MG, Li Z, Blank MA (2002) 14 day endoscopy study comparing risedronate and alendronate in postmenopausal women stratified by Helicobacter pylori status. J Rheumatol 29:1965–1974
Valkenhoef G, Tervonen T, Brock B, Hillege H (2012) Algorithmic parameterization of mixed treatment comparisons. Stat Comput 22:1099–1111
van Staa T, Abenhaim L, Cooper C (1997) Upper gastrointestinal adverse events and cyclical etidronate. Am J Med 103:462–467
Wells GA, Cranney A, Peterson J, Boucher M, Shea B, Robinson V, Coyle D, Tugwell P (2008) Etidronate for the primary and secondary prevention of osteoporotic fractures in postmenopausal women. Cochrane Database Syst Rev CD003376
Wells GA, Cranney A, Peterson J, Boucher M, Shea B, Robinson V, Coyle D, Tugwell P (2008) Alendronate for the primary and secondary prevention of osteoporotic fractures in postmenopausal women. Cochrane Database Syst Rev CD001155
Wells G, Cranney A, Peterson J, Boucher M, Shea B, Robinson V, Coyle D, Tugwell P (2008) Risedronate for the primary and secondary prevention of osteoporotic fractures in postmenopausal women. Cochrane Database Syst Rev CD004523
Citation for papers included in analysis:
Adami S, Passeri M, Ortolani S et al (1995) Effects of oral alendronate and intranasal salmon calcitonin on bone mass and biochemical markers of bone turnover in postmenopausal women with osteoporosis. Bone 17:383–390
Ascott-Evans BH, Guanabens N, Kivinen S, Stuckey BG, Magaril CH, Vandormael K, Stych B, Melton ME (2003) Alendronate prevents loss of bone density associated with discontinuation of hormone replacement therapy: a randomized controlled trial. Arch Intern Med 163:789–794
Bell NH, Bilezikian JP, Bone HG 3rd, Kaur A, Maragoto A, Santora AC (2002) Alendronate increases bone mass and reduces bone markers in postmenopausal African-American women. J Clin Endocrinol Metab 87:2792–2797
Bone HG, Downs RW Jr, Tucci JR et al (1997) Dose–response relationships for alendronate treatment in osteoporotic elderly women. Alendronate Elderly Osteoporosis Study Centers. J Clin Endocrinol Metab 82:265–274
Bone HG, Greenspan SL, McKeever C et al (2000) Alendronate and estrogen effects in postmenopausal women with low bone mineral density. Alendronate/Estrogen Study Group. J Clin Endocrinol Metab 85:720–726
Boonen S, Orwoll ES, Wenderoth D, Stoner KJ, Eusebio R, Delmas PD (2009) Once-weekly risedronate in men with osteoporosis: results of a 2-year, placebo-controlled, double-blind, multicenter study. J Bone Miner Res 24:719–725
Clemmesen B, Ravn P, Zegels B, Taquet AN, Christiansen C, Reginster JY (1997) A 2-year phase II study with 1-year of follow-up of risedronate (NE-58095) in postmenopausal osteoporosis. Osteoporos Int 7:488–495
Cryer B, Binkley N, Simonelli C, Lewiecki EM, Lanza F, Chen E, Petruschke RA, Mullen C, de Papp AE (2005) A randomized, placebo-controlled, 6-month study of once-weekly alendronate oral solution for postmenopausal osteoporosis. Am J Geriatr Pharmacother 3:127–136
Devogelaer JP, Broll H, Correa-Rotter R et al (1996) Oral alendronate induces progressive increases in bone mass of the spine, hip, and total body over 3 years in postmenopausal women with osteoporosis. Bone 18:141–150
Downs RW Jr, Bell NH, Ettinger MP, Walsh BW, Favus MJ, Mako B, Wang L, Smith ME, Gormley GJ, Melton ME (2000) Comparison of alendronate and intranasal calcitonin for treatment of osteoporosis in postmenopausal women. J Clin Endocrinol Metab 85:1783–1788
Fogelman I, Ribot C, Smith R, Ethgen D, Sod E, Reginster JY (2000) Risedronate reverses bone loss in postmenopausal women with low bone mass: results from a multinational, double-blind, placebo-controlled trial. BMD-MN Study Group. J Clin Endocrinol Metab 85:1895–1900
Fujita T, Orimo H, Inoue T et al (2007) Clinical effect of bisphosphonate and vitamin D on osteoporosis: reappraisal of a multicenter double-blind clinical trial comparing etidronate and alfacalcidol. J Bone Miner Metab 25:130–137
Fukunaga M, Kushida K, Kishimoto H et al (2002) A comparison of the effect of risedronate and etidronate on lumbar bone mineral density in Japanese patients with osteoporosis: a randomized controlled trial. Osteoporos Int 13:971–979
Greenspan S, Field-Munves E, Tonino R, Smith M, Petruschke R, Wang L, Yates J, de Papp AE, Palmisano J (2002) Tolerability of once-weekly alendronate in patients with osteoporosis: a randomized, double-blind, placebo-controlled study. Mayo Clin Proc 77:1044–1052
Greenspan SL, Resnick NM, Parker RA (2003) Combination therapy with hormone replacement and alendronate for prevention of bone loss in elderly women: a randomized controlled trial. JAMA 289:2525–2533
Harris ST, Watts NB, Genant HK et al (1999) Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis: a randomized controlled trial. Vertebral Efficacy With Risedronate Therapy (VERT) Study Group. JAMA 282:1344–1352
Hosking D, Adami S, Felsenberg D et al (2003) Comparison of change in bone resorption and bone mineral density with once-weekly alendronate and daily risedronate: a randomised, placebo-controlled study. Curr Med Res Opin 19:383–394
Ilter E, Karalok H, Tufekci EC, Batur O (2006) Efficacy and acceptability of risedronate 5 mg daily compared with 35 mg once weekly for the treatment of postmenopausal osteoporosis. Climacteric 9:129–134
Iwamoto J, Takeda T, Ichimura S (2001) Effect of menatetrenone on bone mineral density and incidence of vertebral fractures in postmenopausal women with osteoporosis: a comparison with the effect of etidronate. J Orthop Sci 6:487–492
Johnell O, Scheele WH, Lu Y, Reginster JY, Need AG, Seeman E (2002) Additive effects of raloxifene and alendronate on bone density and biochemical markers of bone remodeling in postmenopausal women with osteoporosis. J Clin Endocrinol Metab 87:985–992
Kung AW, Yeung SS, Chu LW (2000) The efficacy and tolerability of alendronate in postmenopausal osteoporotic Chinese women: a randomized placebo-controlled study. Calcif Tissue Int 67:286–290
Kushida K, Fukunaga M, Kishimoto H et al (2004) A comparison of incidences of vertebral fracture in Japanese patients with involutional osteoporosis treated with risedronate and etidronate: a randomized, double-masked trial. J Bone Miner Metab 22:469–478
Kushida K, Shiraki M, Nakamura T et al (2004) Alendronate reduced vertebral fracture risk in postmenopausal Japanese women with osteoporosis: a 3-year follow-up study. J Bone Miner Metab 22:462–468
Lau EM, Woo J, Chan YH, Griffith J (2000) Alendronate prevents bone loss in Chinese women with osteoporosis. Bone 27:677–680
Leung JY, Ho AY, Ip TP, Lee G, Kung AW (2005) The efficacy and tolerability of risedronate on bone mineral density and bone turnover markers in osteoporotic Chinese women: a randomized placebo-controlled study. Bone 36:358–364
Lewiecki EM, Miller PD, McClung MR, Cohen SB, Bolognese MA, Liu Y, Wang A, Siddhanti S, Fitzpatrick LA (2007) Two-year treatment with denosumab (AMG 162) in a randomized phase 2 study of postmenopausal women with low BMD. J Bone Miner Res 22:1832–1841
Liberman UA, Weiss SR, Broll J, Minne HW, Quan H, Bell NH, Rodriguez-Portales J, Downs RW Jr, Dequeker J, Favus M (1995) Effect of oral alendronate on bone mineral density and the incidence of fractures in postmenopausal osteoporosis. The Alendronate Phase III Osteoporosis Treatment Study Group. N Engl J Med 333:1437–1443
Lyles KW, Colon-Emeric CS, Magaziner JS et al (2007) Zoledronic acid in reducing clinical fracture and mortality after hip fracture. N Engl J Med 357:nihpa40967
McClung MR, Geusens P, Miller PD et al (2001) Effect of risedronate on the risk of hip fracture in elderly women. Hip Intervention Program Study Group. N Engl J Med 344:333–340
McClung MR, Lewiecki EM, Cohen SB et al (2006) Denosumab in postmenopausal women with low bone mineral density. N Engl J Med 354:821–831
McClung M, Recker R, Miller P, Fiske D, Minkoff J, Kriegman A, Zhou W, Adera M, Davis J (2007) Intravenous zoledronic acid 5 mg in the treatment of postmenopausal women with low bone density previously treated with alendronate. Bone 41:122–128
Murphy MG, Weiss S, McClung M, Schnitzer T, Cerchio K, Connor J, Krupa D, Gertz BJ (2001) Effect of alendronate and MK-677 (a growth hormone secretagogue), individually and in combination, on markers of bone turnover and bone mineral density in postmenopausal osteoporotic women. J Clin Endocrinol Metab 86:1116–1125
Orwoll E, Ettinger M, Weiss S et al (2000) Alendronate for the treatment of osteoporosis in men. N Engl J Med 343:604–610
Orwoll ES, Miller PD, Adachi JD, Brown J, Adler RA, Kendler D, Bucci-Rechtweg C, Readie A, Mesenbrink P, Weinstein RS (2010) Efficacy and safety of a once-yearly i.v. infusion of zoledronic acid 5 mg versus a once-weekly 70-mg oral alendronate in the treatment of male osteoporosis: a randomized, multicenter, double-blind, active-controlled study. J Bone Miner Res 25:2239–2250
Pols HA, Felsenberg D, Hanley DA et al (1999) Multinational, placebo-controlled, randomized trial of the effects of alendronate on bone density and fracture risk in postmenopausal women with low bone mass: results of the FOSIT study. Fosamax International Trial Study Group. Osteoporos Int 9:461–468
Reginster J, Minne HW, Sorensen OH et al (2000) Randomized trial of the effects of risedronate on vertebral fractures in women with established postmenopausal osteoporosis. Vertebral Efficacy with Risedronate Therapy (VERT) Study Group. Osteoporos Int 11:83–91
Reid IR, Brown JP, Burckhardt P et al (2002) Intravenous zoledronic acid in postmenopausal women with low bone mineral density. N Engl J Med 346:653–661
Reid DM, Hosking D, Kendler D, Brandi ML, Wark JD, Weryha G, Marques-Neto JF, Gaines KA, Verbruggen N, Melton ME (2006) Alendronic acid produces greater effects than risedronic acid on bone density and turnover in postmenopausal women with osteoporosis: results of FACTS -international. Clin Drug Investig 26:63–74
Saag K, Lindsay R, Kriegman A, Beamer E, Zhou W (2007) A single zoledronic acid infusion reduces bone resorption markers more rapidly than weekly oral alendronate in postmenopausal women with low bone mineral density. Bone 40:1238–1243
Seeman E, Delmas PD, Hanley DA et al (2010) Microarchitectural deterioration of cortical and trabecular bone: differing effects of denosumab and alendronate. J Bone Miner Res 25:1886–1894
Shiraki M, Fukunaga M, Kushida K et al (2003) A double-blind dose-ranging study of risedronate in Japanese patients with osteoporosis (a study by the Risedronate Late Phase II Research Group). Osteoporos Int 14:225–234
Shiraki M, Kushida K, Fukunaga M et al (1999) A double-masked multicenter comparative study between alendronate and alfacalcidol in Japanese patients with osteoporosis. The Alendronate Phase III Osteoporosis Treatment Research Group. Osteoporos Int 10:183–192
Tucci JR, Tonino RP, Emkey RD, Peverly CA, Kher U, Santora AC 2nd (1996) Effect of three years of oral alendronate treatment in postmenopausal women with osteoporosis. Am J Med 101:488–501
Watts NB, Harris ST, Genant HK et al (1990) Intermittent cyclical etidronate treatment of postmenopausal osteoporosis. N Engl J Med 323:73–79
Acknowledgments
This research was supported by a research grant to Dr. Cadarette from the Ontario Ministry of Research and Innovation Early Researcher Award (ER09-06-043). Dr. Cadarette is supported by a Canadian Institutes of Health Research (CIHR) New Investigator Award in Aging and Osteoporosis (MSH-95364), and Dr. Tadrous is supported by a CIHR Fredrick Banting and Charles Best Canada Graduate Scholarship Doctoral Award (GSD-11342). Dr. Mamdani has served as an advisory board member for the following pharmaceutical companies: Astra Zeneca, Bristol-Myers Squibb, Eli Lilly and Company, Glaxo Smith Kline, Hoffman La Roche, Novartis, Novo Nordisk and Pfizer.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
PDF 551 kb
Rights and permissions
About this article
Cite this article
Tadrous, M., Wong, L., Mamdani, M.M. et al. Comparative gastrointestinal safety of bisphosphonates in primary osteoporosis: a network meta-analysis. Osteoporos Int 25, 1225–1235 (2014). https://doi.org/10.1007/s00198-013-2576-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-013-2576-2