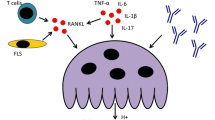
Abstract
The destruction of articular structures in the course of inflammatory arthritides such as rheumatoid arthritis (RA) or seronegative spondyloarthropathies is the most serious direct consequence of these diseases. Indeed, joint damage constitutes the “organ damage” of RA and—just like in all other diseases with organ involvement—such damage will usually be irreversible, cause permanent loss of function and subsequent disability. Research has identified a number of mechanisms and mediators of damage to articular structures such as bone and cartilage, ranging from proinflammatory cytokines, signal transduction pathways and cells types, which will be discussed in this review.

Similar content being viewed by others
References
Anandarajah AP, Schwarz EM (2009) Bone loss in the spondyloarthropathies: role of osteoclast, RANKL, RANK and OPG in the spondyloarthropathies. Adv Exp Med Biol 649:85–99
Kvien TK (2004) Epidemiology and burden of illness of rheumatoid arthritis. Pharmacoeconomics 22(2 Suppl 1):1–12
Schett G, Smolen JS (2005) New insights in the mechanism of bone loss in arthritis. Curr Pharm Des 11(23):3039–3049
Scott DL, Pugner K, Kaarela K, Doyle DV, Woolf A, Holmes J et al (2000) The links between joint damage and disability in rheumatoid arthritis. Rheumatology (Oxford) 39(2):122–132
Scott DL, Smith C, Kingsley G (2003) Joint damage and disability in rheumatoid arthritis: an updated systematic review. Clin Exp Rheumatol 21(5 Suppl 31):S20–S27
Tak PP, Breedveld FC (1999) Current perspectives on synovitis. Arthritis Res 1(1):11–16
Burmester GR, Jahn B, Gramatzki M, Zacher J, Kalden JR (1984) Activated T cells in vivo and in vitro: divergence in expression of Tac and Ia antigens in the nonblastoid small T cells of inflammation and normal T cells activated in vitro. J Immunol 133(3):1230–1234
Klein K, Gay S (2013) Epigenetic modifications in rheumatoid arthritis, a review. Curr Opin Pharmacol 13(3):420–425
Kiener HP, Baghestanian M, Dominkus M, Walchshofer S, Ghannadan M, Willheim M et al (1998) Expression of the C5a receptor (CD88) on synovial mast cells in patients with rheumatoid arthritis. Arthritis Rheum 41(2):233–245
Kiener HP, Hofbauer R, Tohidast-Akrad M, Walchshofer S, Redlich K, Bitzan P et al (2000) Tumor necrosis factor alpha promotes the expression of stem cell factor in synovial fibroblasts and their capacity to induce mast cell chemotaxis. Arthritis Rheum 43(1):164–174
Teitelbaum SL, Ross FP (2003) Genetic regulation of osteoclast development and function. Nat Rev Genet 4(8):638–649
Yasuda H, Shima N, Nakagawa N, Yamaguchi K, Kinosaki M, Mochizuki S et al (1998) Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc Natl Acad Sci U S A 95(7):3597–3602
Koga T, Inui M, Inoue K, Kim S, Suematsu A, Kobayashi E et al (2004) Costimulatory signals mediated by the ITAM motif cooperate with RANKL for bone homeostasis. Nature 428(6984):758–763
Takayanagi H, Kim S, Koga T, Nishina H, Isshiki M, Yoshida H et al (2002) Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev Cell 3(6):889–901
Wang ZQ, Ovitt C, Grigoriadis AE, Mohle-Steinlein U, Ruther U, Wagner EF (1992) Bone and haematopoietic defects in mice lacking c-fos. Nature 360(6406):741–745
Novack DV (2011) Role of NF-kappaB in the skeleton. Cell Res 21(1):169–182
Boyle WJ, Simonet WS, Lacey DL (2003) Osteoclast differentiation and activation. Nature 423(6937):337–342
Cohen SB, Dore RK, Lane NE, Ory PA, Peterfy CG, Sharp JT et al (2008) Denosumab treatment effects on structural damage, bone mineral density, and bone turnover in rheumatoid arthritis: a twelve-month, multicenter, randomized, double-blind, placebo-controlled, phase II clinical trial. Arthritis Rheum 58(5):1299–1309
Ishii M, Egen JG, Klauschen F, Meier-Schellersheim M, Saeki Y, Vacher J et al (2009) Sphingosine-1-phosphate mobilizes osteoclast precursors and regulates bone homeostasis. Nature 458(7237):524–528
Ishii M, Kikuta J, Shimazu Y, Meier-Schellersheim M, Germain RN (2010) Chemorepulsion by blood S1P regulates osteoclast precursor mobilization and bone remodeling in vivo. J Exp Med 207(13):2793–2798
Fujii Y, Hirayama T, Ohtake H, Ono N, Inoue T, Sakurai T et al (2012) Amelioration of collagen-induced arthritis by a novel S1P1 antagonist with immunomodulatory activities. J Immunol 188(1):206–215
Cyster JG, Schwab SR (2012) Sphingosine-1-phosphate and lymphocyte egress from lymphoid organs. Annu Rev Immunol 30:69–94
Stradner MH, Gruber G, Angerer H, Huber V, Setznagl D, Kremser ML et al (2013) Sphingosine 1-phosphate counteracts the effects of interleukin-1beta in human chondrocytes. Arthritis Rheum 65(8):2113–2122
Bluml S, Kirchberger S, Bochkov VN, Kronke G, Stuhlmeier K, Majdic O et al (2005) Oxidized phospholipids negatively regulate dendritic cell maturation induced by TLRs and CD40. J Immunol 175(1):501–508
Bluml S, Zupkovitz G, Kirchberger S, Seyerl M, Bochkov VN, Stuhlmeier K et al (2009) Epigenetic regulation of dendritic cell differentiation and function by oxidized phospholipids. Blood 114(27):5481–5489
Kronke G, Katzenbeisser J, Uderhardt S, Zaiss MM, Scholtysek C, Schabbauer G et al (2009) 12/15-lipoxygenase counteracts inflammation and tissue damage in arthritis. J Immunol 183(5):3383–3389
Zwerina J, Redlich K, Polzer K, Joosten L, Kronke G, Distler J et al (2007) TNF-induced structural joint damage is mediated by IL-1. Proc Natl Acad Sci U S A 104(28):11742–11747
Axmann R, Bohm C, Kronke G, Zwerina J, Smolen J, Schett G (2009) Inhibition of interleukin-6 receptor directly blocks osteoclast formation in vitro and in vivo. Arthritis Rheum 60(9):2747–2756
Kobayashi K, Takahashi N, Jimi E, Udagawa N, Takami M, Kotake S et al (2000) Tumor necrosis factor alpha stimulates osteoclast differentiation by a mechanism independent of the ODF/RANKL-RANK interaction. J Exp Med 191(2):275–286
Korb-Pap A, Stratis A, Muhlenberg K, Niederreiter B, Hayer S, Echtermeyer F et al (2012) Early structural changes in cartilage and bone are required for the attachment and invasion of inflamed synovial tissue during destructive inflammatory arthritis. Ann Rheum Dis 71(6):1004–1011
Lee DM, Kiener HP, Agarwal SK, Noss EH, Watts GF, Chisaka O et al (2007) Cadherin-11 in synovial lining formation and pathology in arthritis. Science 315(5814):1006–1010
Ospelt C, Reedquist KA, Gay S, Tak PP (2011) Inflammatory memories: is epigenetics the missing link to persistent stromal cell activation in rheumatoid arthritis? Autoimmun Rev 10(9):519–524
Pierer M, Muller-Ladner U, Pap T, Neidhart M, Gay RE, Gay S (2003) The SCID mouse model: novel therapeutic targets—lessons from gene transfer. Springer Semin Immunopathol 25(1):65–78
Bartok B, Firestein GS (2010) Fibroblast-like synoviocytes: key effector cells in rheumatoid arthritis. Immunol Rev 233(1):233–255
Weichselbaum A (1878) Die feineren Veränderungen des Gelenkknorpels bei fungöser Synovitis Caries der Gelenkenden. Arch Pathol Anat Physiol Klin Med 73:461–475
Goldring MB, Marcu KB (2009) Cartilage homeostasis in health and rheumatic diseases. Arthritis Res Ther 11(3):224
Rengel Y, Ospelt C, Gay S (2007) Proteinases in the joint: clinical relevance of proteinases in joint destruction. Arthritis Res Ther 9(5):221
Huet G, Flipo RM, Colin C, Janin A, Hemon B, Collyn-d’Hooghe M et al (1993) Stimulation of the secretion of latent cysteine proteinase activity by tumor necrosis factor alpha and interleukin-1. Arthritis Rheum 36(6):772–780
Smolen JS, van der Heijde DM, Keystone EC, van Vollenhoven RF, Goldring MB, Guerette B et al (2013) Association of joint space narrowing with impairment of physical function and work ability in patients with early rheumatoid arthritis: protection beyond disease control by adalimumab plus methotrexate. Ann Rheum Dis 72(7):1156–1162
Karonitsch T, von Dalwigk K, Steiner CW, Bluml S, Steiner G, Kiener HP et al (2012) Interferon signals and monocytic sensitization of the interferon-gamma signaling pathway in the peripheral blood of patients with rheumatoid arthritis. Arthritis Rheum 64(2):400–408
Ziegler-Heitbrock L, Hofer TP (2013) Toward a refined definition of monocyte subsets. Front Immunol 4:23
Kawanaka N, Yamamura M, Aita T, Morita Y, Okamoto A, Kawashima M et al (2002) CD14+, CD16+ blood monocytes and joint inflammation in rheumatoid arthritis. Arthritis Rheum 46(10):2578–2586
Rossol M, Kraus S, Pierer M, Baerwald C, Wagner U (2012) The CD14 (bright) CD16+ monocyte subset is expanded in rheumatoid arthritis and promotes expansion of the Th17 cell population. Arthritis Rheum 64(3):671–677
Seeling M, Hillenhoff U, David JP, Schett G, Tuckermann J, Lux A et al (2013) Inflammatory monocytes and Fcgamma receptor IV on osteoclasts are critical for bone destruction during inflammatory arthritis in mice. Proc Natl Acad Sci U S A 110(26):10729–10734
Bruhl H, Cihak J, Plachy J, Kunz-Schughart L, Niedermeier M, Denzel A et al (2007) Targeting of Gr-1+, CCR2+ monocytes in collagen-induced arthritis. Arthritis Rheum 56(9):2975–2985
Quinones MP, Ahuja SK, Jimenez F, Schaefer J, Garavito E, Rao A et al (2004) Experimental arthritis in CC chemokine receptor 2-null mice closely mimics severe human rheumatoid arthritis. J Clin Invest 113(6):856–866
Kremer JM, Westhovens R, Leon M, Di Giorgio E, Alten R, Steinfeld S et al (2003) Treatment of rheumatoid arthritis by selective inhibition of T-cell activation with fusion protein CTLA4Ig. N Engl J Med 349(20):1907–1915
Evans HG, Gullick NJ, Kelly S, Pitzalis C, Lord GM, Kirkham BW et al (2009) In vivo activated monocytes from the site of inflammation in humans specifically promote Th17 responses. Proc Natl Acad Sci U S A 106(15):6232–6237
Kinne RW, Palombo-Kinne E, Emmrich F (1997) T-cells in the pathogenesis of rheumatoid arthritis villains or accomplices? Biochim Biophys Acta 1360(2):109–141
van der Lubbe PA, Dijkmans BA, Markusse HM, Nassander U, Breedveld FC (1995) A randomized, double-blind, placebo-controlled study of CD4 monoclonal antibody therapy in early rheumatoid arthritis. Arthritis Rheum 38(8):1097–1106
Kouskoff V, Korganow AS, Duchatelle V, Degott C, Benoist C, Mathis D (1996) Organ-specific disease provoked by systemic autoimmunity. Cell 87(5):811–822
Zhu J, Paul WE (2008) CD4 T cells: fates, functions, and faults. Blood 112(5):1557–1569
Ogura H, Murakami M, Okuyama Y, Tsuruoka M, Kitabayashi C, Kanamoto M et al (2008) Interleukin-17 promotes autoimmunity by triggering a positive-feedback loop via interleukin-6 induction. Immunity 29(4):628–636
Kong YY, Feige U, Sarosi I, Bolon B, Tafuri A, Morony S et al (1999) Activated T cells regulate bone loss and joint destruction in adjuvant arthritis through osteoprotegerin ligand. Nature 402(6759):304–309
Sato K, Suematsu A, Okamoto K, Yamaguchi A, Morishita Y, Kadono Y et al (2006) Th17 functions as an osteoclastogenic helper T cell subset that links T cell activation and bone destruction. J Exp Med 203(12):2673–2682
Soderstrom K, Stein E, Colmenero P, Purath U, Muller-Ladner U, de Matos CT et al (2010) Natural killer cells trigger osteoclastogenesis and bone destruction in arthritis. Proc Natl Acad Sci U S A 107(29):13028–13033
Chan A, Filer A, Parsonage G, Kollnberger S, Gundle R, Buckley CD et al (2008) Mediation of the proinflammatory cytokine response in rheumatoid arthritis and spondylarthritis by interactions between fibroblast-like synoviocytes and natural killer cells. Arthritis Rheum 58(3):707–717
Lo CK, Lam QL, Sun L, Wang S, Ko KH, Xu H et al (2008) Natural killer cell degeneration exacerbates experimental arthritis in mice via enhanced interleukin-17 production. Arthritis Rheum 58(9):2700–2711
Leavenworth JW, Wang X, Wenander CS, Spee P, Cantor H (2011) Mobilization of natural killer cells inhibits development of collagen-induced arthritis. Proc Natl Acad Sci U S A 108(35):14584–14589
Bluml S, McKeever K, Ettinger R, Smolen J, Herbst R (2013) B-cell targeted therapeutics in clinical development. Arthritis Res Ther 15(Suppl 1):S4
Bugatti S, Codullo V, Caporali R, Montecucco C (2007) B cells in rheumatoid arthritis. Autoimmun Rev 7(2):137–142
Edwards JC, Szczepanski L, Szechinski J, Filipowicz-Sosnowska A, Emery P, Close DR et al (2004) Efficacy of B-cell-targeted therapy with rituximab in patients with rheumatoid arthritis. N Engl J Med 350(25):2572–2581
Wendling D, Dougados M, Berenbaum F, Brocq O, Schaeverbeke T, Mazieres B et al (2012) Rituximab treatment for spondyloarthritis. A nationwide series: data from the AIR registry of the French Society of Rheumatology. J Rheumatol 39(12):2327–2331
Meyer O, Labarre C, Dougados M, Goupille P, Cantagrel A, Dubois A et al (2003) Anticitrullinated protein/peptide antibody assays in early rheumatoid arthritis for predicting five year radiographic damage. Ann Rheum Dis 62(2):120–126
Mewar D, Coote A, Moore DJ, Marinou I, Keyworth J, Dickson MC et al (2006) Independent associations of anti-cyclic citrullinated peptide antibodies and rheumatoid factor with radiographic severity of rheumatoid arthritis. Arthritis Res Ther 8(4):R128
Harre U, Georgess D, Bang H, Bozec A, Axmann R, Ossipova E et al (2012) Induction of osteoclastogenesis and bone loss by human autoantibodies against citrullinated vimentin. J Clin Invest 122(5):1791–1802
Aringer M, Gunther C, Lee-Kirsch MA (2013) Innate immune processes in lupus erythematosus. Clin Immunol 147(3):216–222
Albert H, Collin M, Dudziak D, Ravetch JV, Nimmerjahn F (2008) In vivo enzymatic modulation of IgG glycosylation inhibits autoimmune disease in an IgG subclass-dependent manner. Proc Natl Acad Sci U S A 105(39):15005–15009
Nandakumar KS, Collin M, Olsen A, Nimmerjahn F, Blom AM, Ravetch JV et al (2007) Endoglycosidase treatment abrogates IgG arthritogenicity: importance of IgG glycosylation in arthritis. Eur J Immunol 37(10):2973–2982
Karsten CM, Kohl J (2012) The immunoglobulin, IgG Fc receptor and complement triangle in autoimmune diseases. Immunobiology 217(11):1067–1079
Yeo L, Toellner KM, Salmon M, Filer A, Buckley CD, Raza K et al (2011) Cytokine mRNA profiling identifies B cells as a major source of RANKL in rheumatoid arthritis. Ann Rheum Dis 70(11):2022–2028
Jimenez-Boj E, Redlich K, Turk B, Hanslik-Schnabel B, Wanivenhaus A, Chott A et al (2005) Interaction between synovial inflammatory tissue and bone marrow in rheumatoid arthritis. J Immunol 175(4):2579–2588
Gortz B, Hayer S, Redlich K, Zwerina J, Tohidast-Akrad M, Tuerk B et al (2004) Arthritis induces lymphocytic bone marrow inflammation and endosteal bone formation. J Bone Mineral Res:Off J Am Soc Bone Mineral Res 19(6):990–998
Zwerina J, Hayer S, Redlich K, Bobacz K, Kollias G, Smolen JS et al (2006) Activation of p38 MAPK is a key step in tumor necrosis factor-mediated inflammatory bone destruction. Arthritis Rheum 54(2):463–472
Lee JC, Laydon JT, McDonnell PC, Gallagher TF, Kumar S, Green D et al (1994) A protein kinase involved in the regulation of inflammatory cytokine biosynthesis. Nature 372(6508):739–746
Page TH, Brown A, Timms EM, Foxwell BM, Ray KP (2010) Inhibitors of p38 suppress cytokine production in rheumatoid arthritis synovial membranes: does variable inhibition of interleukin-6 production limit effectiveness in vivo? Arthritis Rheum 62(11):3221–3231
Hammaker D, Firestein GS (2010) “Go upstream, young man”: lessons learned from the p38 saga. Ann Rheum Dis 69(Suppl 1):i77–i82
Badger AM, Bradbeer JN, Votta B, Lee JC, Adams JL, Griswold DE (1996) Pharmacological profile of SB 203580, a selective inhibitor of cytokine suppressive binding protein/p38 kinase, in animal models of arthritis, bone resorption, endotoxin shock and immune function. J Pharmacol Exp Ther 279(3):1453–1461
Criado G, Risco A, Alsina-Beauchamp D, Perez-Lorenzo MJ, Escos A, Cuenda A (2014) Alternative p38 MAPKs are essential for collagen-induced arthritis. Arthritis Rheumatol 66(5):1208–1217
Mocsai A, Humphrey MB, Van Ziffle JA, Hu Y, Burghardt A, Spusta SC et al (2004) The immunomodulatory adapter proteins DAP12 and Fc receptor gamma-chain (FcRgamma) regulate development of functional osteoclasts through the Syk tyrosine kinase. Proc Natl Acad Sci U S A 101(16):6158–6163
Mocsai A, Ruland J, Tybulewicz VL (2010) The SYK tyrosine kinase: a crucial player in diverse biological functions. Nat Rev Immunol 10(6):387–402
Pine PR, Chang B, Schoettler N, Banquerigo ML, Wang S, Lau A et al (2007) Inflammation and bone erosion are suppressed in models of rheumatoid arthritis following treatment with a novel Syk inhibitor. Clin Immunol 124(3):244–257
Weinblatt ME, Kavanaugh A, Genovese MC, Musser TK, Grossbard EB, Magilavy DB (2010) An oral spleen tyrosine kinase (Syk) inhibitor for rheumatoid arthritis. N Engl J Med 363(14):1303–1312
Scott IC, Scott DL (2014) Spleen tyrosine kinase inhibitors for rheumatoid arthritis: where are we now? Drugs 74(4):415–422
O’Shea JJ, Kontzias A, Yamaoka K, Tanaka Y, Laurence A (2013) Janus kinase inhibitors in autoimmune diseases. Ann Rheum Dis 72(Suppl 2):ii111–ii115
Ghoreschi K, Jesson MI, Li X, Lee JL, Ghosh S, Alsup JW et al (2011) Modulation of innate and adaptive immune responses by tofacitinib (CP-690,550). J Immunol 186(7):4234–4243
Van Rompaey L, Galien R, van der Aar EM, Clement-Lacroix P, Nelles L, Smets B et al (2013) Preclinical characterization of GLPG0634, a selective inhibitor of JAK1, for the treatment of inflammatory diseases. J Immunol 191(7):3568–3577
LaBranche TP, Jesson MI, Radi ZA, Storer CE, Guzova JA, Bonar SL et al (2012) JAK inhibition with tofacitinib suppresses arthritic joint structural damage through decreased RANKL production. Arthritis Rheum 64(11):3531–3542
Rosengren S, Corr M, Firestein GS, Boyle DL (2012) The JAK inhibitor CP-690,550 (tofacitinib) inhibits TNF-induced chemokine expression in fibroblast-like synoviocytes: autocrine role of type I interferon. Ann Rheum Dis 71(3):440–447
Migita K, Komori A, Torigoshi T, Maeda Y, Izumi Y, Jiuchi Y et al (2011) CP690,550 inhibits oncostatin M-induced JAK/STAT signaling pathway in rheumatoid synoviocytes. Arthritis Res Ther 13(3):R72
Kremer JM, Bloom BJ, Breedveld FC, Coombs JH, Fletcher MP, Gruben D et al (2009) The safety and efficacy of a JAK inhibitor in patients with active rheumatoid arthritis: results of a double-blind, placebo-controlled phase IIa trial of three dosage levels of CP-690,550 versus placebo. Arthritis Rheum 60(7):1895–1905
van der Heijde D, Tanaka Y, Fleischmann R, Keystone E, Kremer J, Zerbini C et al (2013) Tofacitinib (CP-690,550) in patients with rheumatoid arthritis receiving methotrexate: twelve-month data from a twenty-four-month phase III randomized radiographic study. Arthritis Rheum 65(3):559–570
Leslie NR, Downes CP (2004) PTEN function: how normal cells control it and tumour cells lose it. Biochem J 382(Pt 1):1–11
Vogt PK, Hart JR, Gymnopoulos M, Jiang H, Kang S, Bader AG et al (2010) Phosphatidylinositol 3-kinase: the oncoprotein. Curr Top Microbiol Immunol 347:79–104
Camps M, Ruckle T, Ji H, Ardissone V, Rintelen F, Shaw J et al (2005) Blockade of PI3Kgamma suppresses joint inflammation and damage in mouse models of rheumatoid arthritis. Nat Med 11(9):936–943
Hayer S, Pundt N, Peters MA, Wunrau C, Kuhnel I, Neugebauer K et al (2009) PI3Kgamma regulates cartilage damage in chronic inflammatory arthritis. FASEB J 23(12):4288–4298
Bluml S, Friedrich M, Lohmeyer T, Sahin E, Saferding V, Brunner J, et al. (2013) Loss of phosphatase and tensin homolog (PTEN) in myeloid cells controls inflammatory bone destruction by regulating the osteoclastogenic potential of myeloid cells. Ann Rheum Dis
Takeshita S, Namba N, Zhao JJ, Jiang Y, Genant HK, Silva MJ et al (2002) SHIP-deficient mice are severely osteoporotic due to increased numbers of hyper-resorptive osteoclasts. Nat Med 8(9):943–949
Zhou P, Kitaura H, Teitelbaum SL, Krystal G, Ross FP, Takeshita S (2006) SHIP1 negatively regulates proliferation of osteoclast precursors via Akt-dependent alterations in D-type cyclins and p27. J Immunol 177(12):8777–8784
Gasparini C, Feldmann M (2012) NF-kappaB as a target for modulating inflammatory responses. Curr Pharm Des 18(35):5735–5745
Hoesel B, Schmid JA (2013) The complexity of NF-kappaB signaling in inflammation and cancer. Mol Cancer 12:86
Lee SW, Kim JH, Park YB, Lee SK (2009) Bortezomib attenuates murine collagen-induced arthritis. Ann Rheum Dis 68(11):1761–1767
Polzer K, Neubert K, Meister S, Frey B, Baum W, Distler JH et al (2011) Proteasome inhibition aggravates tumor necrosis factor-mediated bone resorption in a mouse model of inflammatory arthritis. Arthritis Rheum 63(3):670–680
Yannaki E, Papadopoulou A, Athanasiou E, Kaloyannidis P, Paraskeva A, Bougiouklis D et al (2010) The proteasome inhibitor bortezomib drastically affects inflammation and bone disease in adjuvant-induced arthritis in rats. Arthritis Rheum 62(11):3277–3288
Cejka D, Hayer S, Niederreiter B, Sieghart W, Fuereder T, Zwerina J et al (2010) Mammalian target of rapamycin signaling is crucial for joint destruction in experimental arthritis and is activated in osteoclasts from patients with rheumatoid arthritis. Arthritis Rheum 62(8):2294–2302
Laragione T, Gulko PS (2010) mTOR regulates the invasive properties of synovial fibroblasts in rheumatoid arthritis. Mol Med 16(9–10):352–358
Saxena A, Raychaudhuri SK, Raychaudhuri SP (2011) Interleukin-17-induced proliferation of fibroblast-like synovial cells is mTOR dependent. Arthritis Rheum 63(5):1465–1466
Weichhart T, Costantino G, Poglitsch M, Rosner M, Zeyda M, Stuhlmeier KM et al (2008) The TSC-mTOR signaling pathway regulates the innate inflammatory response. Immunity 29(4):565–577
Monroe DG, McGee-Lawrence ME, Oursler MJ, Westendorf JJ (2012) Update on Wnt signaling in bone cell biology and bone disease. Gene 492(1):1–18
Diarra D, Stolina M, Polzer K, Zwerina J, Ominsky MS, Dwyer D et al (2007) Dickkopf-1 is a master regulator of joint remodeling. Nat Med 13(2):156–163
Dihlmann S, Siermann A, von Knebel DM (2001) The nonsteroidal anti-inflammatory drugs aspirin and indomethacin attenuate beta-catenin/TCF-4 signaling. Oncogene 20(5):645–653
Boon EM, Keller JJ, Wormhoudt TA, Giardiello FM, Offerhaus GJ, van der Neut R et al (2004) Sulindac targets nuclear beta-catenin accumulation and Wnt signalling in adenomas of patients with familial adenomatous polyposis and in human colorectal cancer cell lines. Br J Cancer 90(1):224–229
Wanders A, Heijde D, Landewe R, Behier JM, Calin A, Olivieri I et al (2005) Nonsteroidal antiinflammatory drugs reduce radiographic progression in patients with ankylosing spondylitis: a randomized clinical trial. Arthritis Rheum 52(6):1756–1765
Braun J, Kalden JR (2009) Biologics in the treatment of rheumatoid arthritis and ankylosing spondylitis. Clin Exp Rheumatol 27(4 Suppl 55):S164–S167
van der Heijde D, Salonen D, Weissman BN, Landewe R, Maksymowych WP, Kupper H et al (2009) Assessment of radiographic progression in the spines of patients with ankylosing spondylitis treated with adalimumab for up to 2 years. Arthritis Res Ther 11(4):R127
Bluml S, Scheinecker C, Smolen JS, Redlich K (2012) Targeting TNF receptors in rheumatoid arthritis. Int Immunol 24(5):275–281
Armaka M, Apostolaki M, Jacques P, Kontoyiannis DL, Elewaut D, Kollias G (2008) Mesenchymal cell targeting by TNF as a common pathogenic principle in chronic inflammatory joint and intestinal diseases. J Exp Med 205(2):331–337
Bluml S, Binder NB, Niederreiter B, Polzer K, Hayer S, Tauber S et al (2010) Antiinflammatory effects of tumor necrosis factor on hematopoietic cells in a murine model of erosive arthritis. Arthritis Rheum 62(6):1608–1619
Binder NB, Puchner A, Niederreiter B, Hayer S, Leiss H, Bluml S et al (2013) Tumor necrosis factor-inhibiting therapy preferentially targets bone destruction but not synovial inflammation in a tumor necrosis factor-driven model of rheumatoid arthritis. Arthritis Rheum 65(3):608–617
Takagi N, Mihara M, Moriya Y, Nishimoto N, Yoshizaki K, Kishimoto T et al (1998) Blockage of interleukin-6 receptor ameliorates joint disease in murine collagen-induced arthritis. Arthritis Rheum 41(12):2117–2121
Schoels MM, van der Heijde D, Breedveld FC, Burmester GR, Dougados M, Emery P et al (2013) Blocking the effects of interleukin-6 in rheumatoid arthritis and other inflammatory rheumatic diseases: systematic literature review and meta-analysis informing a consensus statement. Ann Rheum Dis 72(4):583–589
Smolen JS, Beaulieu A, Rubbert-Roth A, Ramos-Remus C, Rovensky J, Alecock E et al (2008) Effect of interleukin-6 receptor inhibition with tocilizumab in patients with rheumatoid arthritis (OPTION study): a double-blind, placebo-controlled, randomised trial. Lancet 371(9617):987–997
Ohshima S, Saeki Y, Mima T, Sasai M, Nishioka K, Nomura S et al (1998) Interleukin 6 plays a key role in the development of antigen-induced arthritis. Proc Natl Acad Sci U S A 95(14):8222–8226
Kishimoto T (2006) Interleukin-6: discovery of a pleiotropic cytokine. Arthritis Res Ther 8(Suppl 2):S2
Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ et al (2006) The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell 126(6):1121–1133
Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B (2006) TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity 24(2):179–189
Tamura T, Udagawa N, Takahashi N, Miyaura C, Tanaka S, Yamada Y et al (1993) Soluble interleukin-6 receptor triggers osteoclast formation by interleukin 6. Proc Natl Acad Sci U S A 90(24):11924–11928
Smolen JS, Avila JC, Aletaha D (2012) Tocilizumab inhibits progression of joint damage in rheumatoid arthritis irrespective of its anti-inflammatory effects: disassociation of the link between inflammation and destruction. Ann Rheum Dis 71(5):687–693
Joosten LA, Helsen MM, Saxne T, van De Loo FA, Heinegard D, van Den Berg WB (1999) IL-1 alpha beta blockade prevents cartilage and bone destruction in murine type II collagen-induced arthritis, whereas TNF-alpha blockade only ameliorates joint inflammation. J Immunol 163(9):5049–5055
Saijo S, Asano M, Horai R, Yamamoto H, Iwakura Y (2002) Suppression of autoimmune arthritis in interleukin-1-deficient mice in which T cell activation is impaired due to low levels of CD40 ligand and OX40 expression on T cells. Arthritis Rheum 46(2):533–544
Chung Y, Chang SH, Martinez GJ, Yang XO, Nurieva R, Kang HS et al (2009) Critical regulation of early Th17 cell differentiation by interleukin-1 signaling. Immunity 30(4):576–587
Dinarello CA (2011) Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood 117(14):3720–3732
Singh JA, Christensen R, Wells GA, Suarez-Almazor ME, Buchbinder R, Lopez-Olivo MA et al (2009) Biologics for rheumatoid arthritis: an overview of Cochrane reviews. Cochrane Database Syst Rev 4:CD007848
Yao Z, Fanslow WC, Seldin MF, Rousseau AM, Painter SL, Comeau MR et al (1995) Herpesvirus Saimiri encodes a new cytokine, IL-17, which binds to a novel cytokine receptor. Immunity 3(6):811–821
Nakae S, Nambu A, Sudo K, Iwakura Y (2003) Suppression of immune induction of collagen-induced arthritis in IL-17-deficient mice. J Immunol 171(11):6173–6177
Nakae S, Saijo S, Horai R, Sudo K, Mori S, Iwakura Y (2003) IL-17 production from activated T cells is required for the spontaneous development of destructive arthritis in mice deficient in IL-1 receptor antagonist. Proc Natl Acad Sci U S A 100(10):5986–5990
Fossiez F, Djossou O, Chomarat P, Flores-Romo L, Ait-Yahia S, Maat C et al (1996) T cell interleukin-17 induces stromal cells to produce proinflammatory and hematopoietic cytokines. J Exp Med 183(6):2593–2603
Chabaud M, Fossiez F, Taupin JL, Miossec P (1998) Enhancing effect of IL-17 on IL-1-induced IL-6 and leukemia inhibitory factor production by rheumatoid arthritis synoviocytes and its regulation by Th2 cytokines. J Immunol 161(1):409–414
Kotake S, Udagawa N, Takahashi N, Matsuzaki K, Itoh K, Ishiyama S et al (1999) IL-17 in synovial fluids from patients with rheumatoid arthritis is a potent stimulator of osteoclastogenesis. J Clin Invest 103(9):1345–1352
Kikuta J, Wada Y, Kowada T, Wang Z, Sun-Wada GH, Nishiyama I et al (2013) Dynamic visualization of RANKL and Th17-mediated osteoclast function. J Clin Invest 123(2):866–873
Genovese MC, Durez P, Richards HB, Supronik J, Dokoupilova E, Mazurov V et al (2013) Efficacy and safety of secukinumab in patients with rheumatoid arthritis: a phase II, dose-finding, double-blind, randomised, placebo controlled study. Ann Rheum Dis 72(6):863–869
Genovese MC, Greenwald M, Cho CS, Berman A, Jin L, Cameron GS et al (2014) A phase II randomized study of subcutaneous ixekizumab, an anti-interleukin-17 monoclonal antibody, in rheumatoid arthritis patients who were naive to biologic agents or had an inadequate response to tumor necrosis factor inhibitors. Arthritis Rheumatol 66(7):1693–1704
Martin DA, Churchill M, Flores-Suarez L, Cardiel MH, Wallace D, Martin R et al (2013) A phase Ib multiple ascending dose study evaluating safety, pharmacokinetics, and early clinical response of brodalumab, a human anti-IL-17R antibody, in methotrexate-resistant rheumatoid arthritis. Arthritis Res Ther 15(5):R164
Partsch G, Steiner G, Leeb BF, Dunky A, Broll H, Smolen JS (1997) Highly increased levels of tumor necrosis factor-alpha and other proinflammatory cytokines in psoriatic arthritis synovial fluid. J Rheumatol 24(3):518–523
Author information
Authors and Affiliations
Corresponding author
Additional information
This submission is related to Mechanisms of Tissue Injury in Autoimmune Diseases - Dr Dan Eilat
Rights and permissions
About this article
Cite this article
Blüml, S., Redlich, K. & Smolen, J.S. Mechanisms of tissue damage in arthritis. Semin Immunopathol 36, 531–540 (2014). https://doi.org/10.1007/s00281-014-0442-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00281-014-0442-8