Abstract
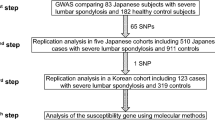
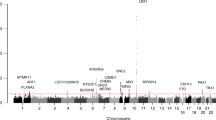
Ossification of the posterior longitudinal ligament of the spine (OPLL) is a common spinal disorder among the elderly that causes myelopathy and radiculopathy. To identify genetic factors for OPLL, we performed a genome-wide association study (GWAS) in ∼8,000 individuals followed by a replication study using an additional ∼7,000 individuals. We identified six susceptibility loci for OPLL: 20p12.3 (rs2423294: P = 1.10 × 10−13), 8q23.1 (rs374810: P = 1.88 × 10−13), 12p11.22 (rs1979679: P = 4.34 × 10−12), 12p12.2 (rs11045000: P = 2.95 × 10−11), 8q23.3 (rs13279799: P = 1.28 × 10−10) and 6p21.1 (rs927485: P = 9.40 × 10−9). Analyses of gene expression in and around the loci suggested that several genes are involved in OPLL etiology through membranous and/or endochondral ossification processes. Our results bring new insight to the etiology of OPLL.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Matsunaga, S. & Sakou, T. in OPLL: Ossification of the Posterior Longitudinal Ligament 2nd edn. (eds. Yonenobu, K., Nakamura, K. & Toyama, Y.) 11–17 (Springer, Tokyo, 2006).
Matsunaga, S. et al. Pathogenesis of myelopathy in patients with ossification of the posterior longitudinal ligament. J. Neurosurg. 96, 168–172 (2002).
Sakou, T., Matsunaga, S. & Koga, H. Recent progress in the study of pathogenesis of ossification of the posterior longitudinal ligament. J. Orthop. Sci. 5, 310–315 (2000).
Taketomi, E., Sakou, T., Matsunaga, S. & Yamaguchi, M. Family study of a twin with ossification of the posterior longitudinal ligament in the cervical spine. Spine 17, S55–S56 (1992).
Okawa, A. et al. Mutation in Npps in a mouse model of ossification of the posterior longitudinal ligament of the spine. Nat. Genet. 19, 271–273 (1998).
Koga, H. et al. Restriction fragment length polymorphism of genes of the α2(XI) collagen, bone morphogenetic protein-2, alkaline phosphatase, and tumor necrosis factor-α among patients with ossification of posterior longitudinal ligament and controls from the Japanese population. Spine 21, 469–473 (1996).
Nakamura, I. et al. Association of the human NPPS gene with ossification of the posterior longitudinal ligament of the spine (OPLL). Hum. Genet. 104, 492–497 (1999).
Tanaka, T. et al. Genomewide linkage and linkage disequilibrium analyses identify COL6A1, on chromosome 21, as the locus for ossification of the posterior longitudinal ligament of the spine. Am. J. Hum. Genet. 73, 812–822 (2003).
Horikoshi, T. et al. A large-scale genetic association study of ossification of the posterior longitudinal ligament of the spine. Hum. Genet. 119, 611–616 (2006).
Yamaguchi-Kabata, Y. et al. Japanese population structure, based on SNP genotypes from 7003 individuals compared to other ethnic groups: effects on population-based association studies. Am. J. Hum. Genet. 83, 445–456 (2008).
Kim, K.A. et al. R-spondin proteins: a novel link to β-catenin activation. Cell Cycle 5, 23–26 (2006).
Monroe, D.G., McGee-Lawrence, M.E., Oursler, M.J. & Westendorf, J.J. Update on Wnt signaling in bone cell biology and bone disease. Gene 492, 1–18 (2012).
Abed, É. et al. R-spondins are newly recognized players in osteoarthritis that regulate Wnt signaling in osteoblasts. Arthritis Rheum. 63, 3865–3875 (2011).
Chaudhuri, J., Chowdhury, D. & Maitra, U. Distinct functions of eukaryotic translation initiation factors eIF1A and eIF3 in the formation of the 40 S ribosomal preinitiation complex. J. Biol. Chem. 274, 17975–17980 (1999).
Christianson, J.C. et al. Defining human ERAD networks through an integrative mapping strategy. Nat. Cell Biol. 14, 93–105 (2012).
Sato, R. et al. Ossification of the posterior longitudinal ligament of the cervical spine: histopathological findings around the calcification and ossification front. J. Neurosurg. Spine 7, 174–183 (2007).
Zhang, N., Kaur, R., Akhter, S. & Legerski, R.J. Cdc5L interacts with ATR and is required for the S-phase cell-cycle checkpoint. EMBO Rep. 10, 1029–1035 (2009).
Ajuh, P. et al. Functional analysis of the human CDC5L complex and identification of its components by mass spectrometry. EMBO J. 19, 6569–6581 (2000).
Yu, J., Madison, J.M., Mundlos, S., Winston, F. & Olsen, B.R. Characterization of a human homologue of the Saccharomyces cerevisiae transcription factor Spt3 (SUPT3H). Genomics 53, 90–96 (1998).
Estrada, K. et al. Genome-wide meta-analysis identifies 56 bone mineral density loci and reveals 14 loci associated with risk of fracture. Nat. Genet. 44, 491–501 (2012).
Karasugi, T. et al. A genome-wide sib-pair linkage analysis of ossification of the posterior longitudinal ligament of the spine. J. Bone Miner. Metab. 31, 136–143 (2013).
Kawaguchi, H. et al. in OPLL: Ossification of the Posterior Longitudinal Ligament 2nd edn. (eds. Yonenobu, K., Nakamura, K. & Toyama, Y.) 71–75 (Springer, Tokyo, 2006).
Shukunami, C. et al. Chondrogenic differentiation of clonal mouse embryonic cell line ATDC5 in vitro: differentiation-dependent gene expression of parathyroid hormone (PTH)/PTH-related peptide receptor. J. Cell Biol. 133, 457–468 (1996).
FANTOM Consortium and RIKEN PMI and CLST (DGT). A promoter-level mammalian expression atlas. Nature 507, 462–470 (2014).
Castleman, V.H. et al. Mutations in radial spoke head protein genes RSPH9 and RSPH4A cause primary ciliary dyskinesia with central-microtubular-pair abnormalities. Am. J. Hum. Genet. 84, 197–209 (2009).
Huber, C. & Cormier-Daire, V. Ciliary disorder of the skeleton. Am. J. Med. Genet. C. Semin. Med. Genet. 160C, 165–174 (2012).
Cornils, H., Kohler, R.S., Hergovich, A. & Hemmings, B.A. Human NDR kinases control G1/S cell cycle transition by directly regulating p21 stability. Mol. Cell. Biol. 31, 1382–1395 (2011).
Harada, Y. et al. Osteogenic lineage commitment of mesenchymal stem cells from patients with ossification of the posterior longitudinal ligament. Biochem. Biophys. Res. Commun. 443, 1014–1020 (2014).
Yang, T.P. et al. Genevar: a database and Java application for the analysis and visualization of SNP-gene associations in eQTL studies. Bioinformatics 26, 2474–2476 (2010).
Raychaudhuri, S. et al. Identifying relationships among genomic disease regions: predicting genes at pathogenic SNP associations and rare deletions. PLoS Genet. 5, e1000534 (2009).
Nakamura, Y. The BioBank Japan Project. Clin. Adv. Hematol. Oncol. 5, 696–697 (2007).
Price, A.L. et al. Principal components analysis corrects for stratification in genome-wide association studies. Nat. Genet. 38, 904–909 (2006).
Breslow, N.E. & Day, N.E. Statistical methods in cancer research. Volume II—the design and analysis of cohort studies. IARC Sci. Publ. (82), 1–406 (1987).
Pruim, R.J. et al. LocusZoom: regional visualization of genome-wide association scan results. Bioinformatics 26, 2336–2337 (2010).
Purcell, S. et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 81, 559–575 (2007).
Tukiainen, T. et al. Chromosome X–wide association study identifies loci for fasting insulin and height and evidence for incomplete dosage compensation. PLoS Genet. 10, e1004127 (2014).
Howie, B., Fuchsberger, C., Stephens, M., Marchini, J. & Abecasis, G.R. Fast and accurate genotype imputation in genome-wide association studies through pre-phasing. Nat. Genet. 44, 955–959 (2012).
1000 Genomes Project Consortium. A map of human genome variation from population-scale sequencing. Nature 467, 1061–1073 (2010).
Li, Y., Willer, C.J., Ding, J., Scheet, P. & Abecasis, G.R. MaCH: using sequence and genotype data to estimate haplotypes and unobserved genotypes. Genet. Epidemiol. 34, 816–834 (2010).
Acknowledgements
We thank the individuals with OPLL and their families who participated in this study and the Japan OPLL Network (Zen-Sekichu-Ren), including Ishikawa prefecture OPLL Tomo-no-kai. We also thank Y. Takahashi and T. Oguma for technical assistance. The work reported in this article was supported by grants from the Ministry of Health, Labour and Welfare of Japan: Committee for Study of Ossification of Spinal Ligament (H23-Nanchi-Ippan-032) and Committee for Research and Development of Therapies for Ossification of Posterior Longitudinal Ligament (H26-Itaku(Nan)-Ippan-053).
Author information
Authors and Affiliations
Consortia
Contributions
S.I. designed the project and provided overall project management. M.N. and S.I. drafted the manuscript. M.N. and M. Kubo performed the genotyping for the GWAS. A.T. analyzed the GWAS data. T. Tsuji, T. Karasugi, H.B., K.U., S. Kawabata, A.O., S.S., K.T., Y. Taniguchi, S.M., M. Kashii, A.S., H.N., Y.K., S.F., M. Takahata, T. Tanaka, K.W., K.K., T. Kanchiku, Z.I., K.M., T. Kaito, S. Kobayashi, K.Y., M. Takahashi, K.C., M.M., K.-I.F. and Y. Toyama managed the DNA samples and clinical data.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
A full list of members and affiliations appears in the Supplementary Note.
Integrated supplementary information
Supplementary Figure 1 Evaluation of the GWAS for OPLL.
(a,b) Principal-component analysis of the GWAS samples with (a) and without (b) four HapMap populations. YRI, Yoruba in Ibadan, Nigeria; CEU, Utah residents with ancestry from northern and western Europe; CHB, Han Chinese in Beijing, China; JPT, Japanese in Tokyo, Japan. (c) A quantile-quantile plot with Cochran-Armitage trend P values from the GWAS. Horizontal and vertical lines represent expected P values under a null distribution and observed P values, respectively. The genetic inflation factor λ is 1.01, indicating that population stratification is unlikely.
Supplementary Figure 2 Conditional logistic regression analysis of six susceptibility loci for OPLL.
Each plot shows the –log10 (P value) against the chromosomal position of each SNP with (red) and without (blue) adjustments for each primary associated SNP indicated in the panel. (a) 20p12.3. (b) 8q23.1. (c) 12p11.22. (d) 12p12.2. (e) 8q23.3. (f) 6p21.1. Each region consisted of a single locus.
Supplementary Figure 3 Regional association plots for the previously reported linkage regions.
Each plot shows the –log10 (P value) against the chromosomal position of each genotyped SNP in the previously reported linkage regions. Each top SNP at five loci (labeled by rs number) is shown with its P value. (a) 1p21. (b) 2p22-2p24. (c) 7q22. (d) 16q24. (e) 20p12. The 20p12 linkage region contained a significantly associated (P < 5 × 10−8) SNP in the GWAS.
Supplementary Figure 4 Expression of genes within the LD blocks of associated SNPs in osteoblasts and fibroblasts.
Gene expression was examined by RT-PCR on mRNA obtained from bone and skin samples from three individuals each. EIF3E, EMC2 and CCDC91 were abundantly expressed in both osteoblasts and fibroblasts. NC, negative control; PC, positive control.
Supplementary Figure 5 Expression of genes within the LD blocks of the associated SNPs during chondrogenic differentiation of ATDC5 cells.
ATDC5 cells were cultured in the presence (filled box) or absence (open box) of chondrogenic differentiation medium (insulin-transferrin-sodium selenite) for the indicated time periods. Expression of Hao1 (a), Rspo2 (b), Eif3e (c), Emc2 (d), Ccdc91 (e), Acan (f), Col2a1 (g) and Sox9 (h) was examined by quantitative PCR. Expression of Hao1, Rspo2 and Ccdc91 was decreased by the induction of chondrogenesis. Data represent mean ± s.d. (n = 3). *P < 0.05 at the same time point.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–5, Supplementary Tables 1–7 and Supplementary Note. (PDF 3107 kb)
Rights and permissions
About this article
Cite this article
Nakajima, M., Takahashi, A., Tsuji, T. et al. A genome-wide association study identifies susceptibility loci for ossification of the posterior longitudinal ligament of the spine. Nat Genet 46, 1012–1016 (2014). https://doi.org/10.1038/ng.3045
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng.3045
This article is cited by
-
A mutation in CCDC91, Homo sapiens coiled-coil domain containing 91 protein, cause autosomal-dominant acrokeratoelastoidosis
European Journal of Human Genetics (2024)
-
Genetics implicates overactive osteogenesis in the development of diffuse idiopathic skeletal hyperostosis
Nature Communications (2023)
-
Genetics of Diffuse Idiopathic Skeletal Hyperostosis and Ossification of the Spinal Ligaments
Current Osteoporosis Reports (2023)
-
Small extracellular vesicle-mediated miR-320e transmission promotes osteogenesis in OPLL by targeting TAK1
Nature Communications (2022)
-
Strong relationship between dyslipidemia and the ectopic ossification of the spinal ligaments
Scientific Reports (2022)