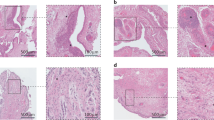
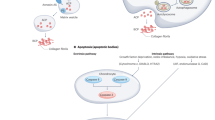
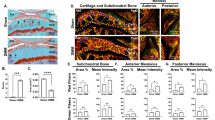
Abstract
Osteoarthritis (OA) involves all the structures of the joint. How the disease is initiated and what factors trigger the disease process remain unclear, although the mechanical environment seems to have a role. Our understanding of the biology of the disease has been hampered by the lack of access to tissue samples from patients with early stage disease, because clinically recognizable symptoms appear late in the osteoarthritic process. However, new data about the early processes in articular cartilage and new tools to identify the early stages of OA are providing fresh insights into the pathological sequence of events. The progressive destruction of cartilage involves degradation of matrix constituents, and rather active, yet inefficient, repair attempts. The release of fragmented molecules provides opportunities to monitor the disease process in patients, and to investigate whether these fragments are involved in propagating OA, for example, by inducing inflammation. The role of bone has not been fully elucidated, but changes in bone seem to be secondary to alterations in articular cartilage, which change the mechanical environment of the bone cells and induce them, in turn, to modulate tissue structure.
Key Points
-
Mechanical factors have a central role in the development of osteoarthritis and in cartilage breakdown; these processes can be modulated by variable load
-
Inflammation is an early and persistent event that might impair the ability of joint tissues to handle mechanical loads
-
Cartilage changes are early events that are coupled, via mechanical or soluble factors, to bone alterations
-
Involvement of tissues in osteoarthritis can be monitored by quantifying levels of released fragments of tissue matrix molecules and inflammation can be assessed by markers such as activated complement factors or cytokines
-
The results of tissue-destructive processes can be visualized by direct joint inspection in arthroscopy, as well as by imaging technology, particularly MRI, ultrasonography and radiography
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Day, J. S. et al. Adaptation of subchondral bone in osteoarthritis. Biorheology 41, 359–368 (2004).
Hunter, D. J., Le Graverand, M. P. & Eckstein, F. Radiologic markers of osteoarthritis progression. Curr. Opin. Rheumatol. 21, 110–117 (2009).
Schneider, E. et al. Magnetic resonance imaging evaluation of weight-bearing subchondral trabecular bone in the knee. Skeletal Radiol. doi:10.1007/s00256-010-0943-z.
Kawcak, C. E., Frisbie, D. D., Werpy, N. M., Park, R. D. & McIlwraith, C. W. Effects of exercise vs experimental osteoarthritis on imaging outcomes. Osteoarthritis Cartilage 16, 1519–1525 (2008).
Englund, M. et al. Meniscal pathology on MRI increases the risk for both incident and enlarging subchondral bone marrow lesions of the knee: the MOST Study. Ann. Rheum. Dis. 69, 1796–1802 (2010).
Saxne, T., Lindell, M., Månsson, B., Petersson, I. F. & Heinegård, D. Inflammation is a feature of the disease process in early knee joint osteoarthritis. Rheumatology (Oxford) 42, 903–904 (2003).
Lotz, M. K. New developments in osteoarthritis. Posttraumatic osteoarthritis: pathogenesis and pharmacological treatment options. Arthritis Res. Ther. 12, 211 (2010).
Metsäranta, M. et al. Chondrodysplasia in transgenic mice harboring a 15-amino acid deletion in the triple helical domain of proα1(II) collagen chain 895. J. Cell Biol. 118, 203–212 (1992).
Jakkula, E. et al. The role of sequence variations within the genes encoding collagen II, IX and XI in non-syndromic, early-onset osteoarthritis. Osteoarthritis Cartilage 13, 497–507 (2005).
Kuivaniemi, H., Tromp, G. & Prockop, D. J. Mutations in fibrillar collagens (types I, II, III, and XI), fibril-associated collagen (type IX), and network-forming collagen (type X) cause a spectrum of diseases of bone, cartilage, and blood vessels. Hum. Mutat. 9, 300–315 (1997).
Dreier, R., Opolka, A., Grifka, J., Bruckner, P. & Grassel, S. Collagen IX-deficiency seriously compromises growth cartilage development in mice. Matrix Biol. 27, 319–329 (2008).
Fässler, R. et al. Mice lacking α1(IX) collagen develop noninflammatory degenerative joint disease. Proc. Natl Acad. Sci. USA 91, 5070–5074 (1994).
Kennedy, J. et al. Novel and recurrent mutations in the C-terminal domain of COMP cluster in two distinct regions and result in a spectrum of phenotypes within the pseudoachondroplasia--multiple epiphyseal dysplasia disease group. Hum. Mutat. 25, 593–594 (2005).
Deere, M., Sanford, T., Francomano, C. A., Daniels, K. & Hecht, J. T. Identification of nine novel mutations in cartilage oligomeric matrix protein in patients with pseudoachondroplasia and multiple epiphyseal dysplasia. Am. J. Med. Genet. 85, 486–490 (1999).
Otten, C., Wagener, R., Paulsson, M. & Zaucke, F. Matrilin-3 mutations that cause chondrodysplasias interfere with protein trafficking while a mutation associated with hand osteoarthritis does not. J. Med. Genet. 42, 774–779 (2005).
Basser, P. J., Schneiderman, R., Bank, R. A., Wachtel, E. & Maroudas, A. Mechanical properties of the collagen network in human articular cartilage as measured by osmotic stress technique. Arch. Biochem. Biophys. 351, 207–219 (1998).
Muir, H., Bullough, P. & Maroudas, A. The distribution of collagen in human articular cartilage with some of its physiological implications. J. Bone Joint Surg. [Br.] 52, 554–563 (1970).
Bennell, K. L. et al. Bone marrow lesions are related to dynamic knee loading in medial knee osteoarthritis. Ann. Rheum. Dis. 69, 1151–1154 (2010).
Kerin, A., Patwari, P., Kuettner, K., Cole, A. & Grodzinsky, A. Molecular basis of osteoarthritis: biomechanical aspects. Cell. Mol. Life Sci. 59, 27–35 (2002).
Englund, M. et al. Meniscal tear in knees without surgery and the development of radiographic osteoarthritis among middle-aged and elderly persons: The Multicenter Osteoarthritis Study. Arthritis Rheum. 60, 831–839 (2009).
Neuman, P. et al. Patellofemoral osteoarthritis 15 years after anterior cruciate ligament injury-—a prospective cohort study. Osteoarthritis Cartilage 17, 284–290 (2009).
Burr, D. B. & Radin, E. L. Microfractures and microcracks in subchondral bone: are they relevant to osteoarthrosis? Rheum. Dis. Clin. North Am. 29, 675–685 (2003).
Radin, E. L. et al. Mechanical determinants of osteoarthrosis. Semin. Arthritis Rheum. 21, 12–21 (1991).
Inerot, S., Heinegård, D., Olsson, S. E., Telhag, H. & Audell, L. Proteoglycan alterations during developing experimental osteoarthritis in a novel hip joint model. J. Orthop. Res. 9, 658–673 (1991).
Palmoski, M. J. & Brandt, K. D. Immobilization of the knee prevents osteoarthritis after anterior cruciate ligament transection. Arthritis Rheum. 25, 1201–1208 (1982).
Segal, R. et al. The impact of hemiparalysis on the expression of osteoarthritis. Arthritis Rheum. 41, 2249–2256 (1998).
Spector, T. D. et al. Low-level increases in serum C-reactive protein are present in early osteoarthritis of the knee and predict progressive disease. Arthritis Rheum. 40, 723–727 (1997).
Sellam, J. & Berenbaum, F. The role of synovitis in pathophysiology and clinical symptoms of osteoarthritis. Nat. Rev. Rheumatol. 6, 625–635 (2010).
Saklatvala, J. Characterization of catabolin, the major product of pig synovial tissue that induces resorption of cartilage proteoglycan in vitro. Biochem. J. 199, 705–714 (1981).
Gruber, J. et al. Induction of interleukin-1 in articular cartilage by explantation and cutting. Arthritis Rheum. 50, 2539–2546 (2004).
Saklatvala, J. Interleukin 1: purification and biochemical aspects of its action on cartilage. J. Rheumatol. 14, 52–54 (1987).
Pratta, M. A., Di Meo, T. M., Ruhl, D. M. & Arner, E. C. Effect of interleukin-1-β and tumor necrosis factor-α on cartilage proteoglycan metabolism in vitro. Agents Actions 27, 250–253 (1989).
Sjöberg, A., Önnerfjord, P., Mörgelin, M., Heinegård, D. & Blom, A. M. The extracellular matrix and inflammation: fibromodulin activates the classical pathway of complement by directly binding C1q. J. Biol. Chem. 280, 32301–32308 (2005).
Sjöberg, A. P. et al. Short leucine-rich glycoproteins of the extracellular matrix display diverse patterns of complement interaction and activation. Mol. Immunol. 46, 830–839 (2009).
Groeneveld, T. W. et al. Interactions of the extracellular matrix proteoglycans decorin and biglycan with C1q and collectins. J. Immunol. 175, 4715–4723 (2005).
Krumdieck, R., Hook, M., Rosenberg, L. C. & Volanakis, J. E. The proteoglycan decorin binds C1q and inhibits the activity of the C1 complex. J. Immunol. 149, 3695–3701 (1992).
Happonen, K. E. et al. Regulation of complement by COMP allows for a novel molecular diagnostic principle in rheumatoid arthritis. Arthritis Rheum. doi: 10.1002/art.27720.
Verrouil, E. & Mazieres, B. Etiologic factors in finger osteoarthritis. Rev. Rhum. Engl. Ed. 62, 9S–13S (1995).
Janusz, M. J. et al. Induction of osteoarthritis in the rat by surgical tear of the meniscus: Inhibition of joint damage by a matrix metalloproteinase inhibitor. Osteoarthritis Cartilage 10, 785–791 (2002).
Libicher, M., Ivancic, M., Hoffmann, M. & Wenz, W. Early changes in experimental osteoarthritis using the Pond-Nuki dog model: technical procedure and initial results of in vivo MR imaging. Eur. Radiol. 15, 390–394 (2005).
Lorenzo, P., Bayliss, M. T. & Heinegård, D. Altered patterns and synthesis of extracellular matrix macromolecules in early osteoarthritis. Matrix Biol. 23, 381–391 (2004).
Kshirsagar, A. A., Watson, P. J., Tyler, J. A. & Hall, L. D. Measurement of localized cartilage volume and thickness of human knee joints by computer analysis of three-dimensional magnetic resonance images. Invest. Radiol. 33, 289–299 (1998).
Glasson, S. S. et al. Deletion of active ADAMTS5 prevents cartilage degradation in a murine model of osteoarthritis. Nature 434, 644–648 (2005).
Lark, M. W. et al. Aggrecan degradation in human cartilage. Evidence for both matrix metalloproteinase and aggrecanase activity in normal, osteoarthritic, and rheumatoid joints. J. Clin. Invest. 100, 93–106 (1997).
Lohmander, L. S., Neame, P. J. & Sandy, J. D. The structure of aggrecan fragments in human synovial fluid. Evidence that aggrecanase mediates cartilage degradation in inflammatory joint disease, joint injury, and osteoarthritis. Arthritis Rheum. 36, 1214–1222 (1993).
Pratta, M. A. et al. Development and characterization of a highly specific and sensitive sandwich ELISA for detection of aggrecanase-generated aggrecan fragments. Osteoarthritis Cartilage 14, 702–713 (2006).
Sandy, J. D. & Verscharen, C. Analysis of aggrecan in human knee cartilage and synovial fluid indicates that aggrecanase (ADAMTS) activity is responsible for the catabolic turnover and loss of whole aggrecan whereas other protease activity is required for C-terminal processing in vivo. Biochem. J. 358, 615–626 (2001).
Lorenzo, P., Bayliss, M. T. & Heinegård, D. A novel cartilage protein (CILP) present in the mid-zone of human articular cartilage increases with age. J. Biol. Chem. 273, 23463–23468 (1998).
Lorenzo, P. et al. Identification and characterization of asporin. A novel member of the leucine rich repeat protein family closely related to decorin and biglycan. J. Biol. Chem. 276, 12201–12211 (2001).
Stanton, H. et al. ADAMTS5 is the major aggrecanase in mouse cartilage in vivo and in vitro. Nature 434, 648–652 (2005).
Glasson, S. S. et al. Characterization of and osteoarthritis susceptibility in ADAMTS-4-knockout mice. Arthritis Rheum. 50, 2547–2558 (2004).
Sandy, J. D., Thompson, V., Doege, K. & Verscharen, C. The intermediates of aggrecanase-dependent cleavage of aggrecan in rat chondrosarcoma cells treated with interleukin-1. Biochem. J. 351, 161–166 (2000).
Bank, R. A., Soudry, M., Maroudas, A., Mizrahi, J. & Tekoppele, J. M. The increased swelling and instantaneous deformation of osteoarthritic cartilage is highly correlated with collagen degradation. Arthritis Rheum. 43, 2202–2210 (2000).
Arokoski, J., Kiviranta, I., Jurvelin, J., Tammi, M. & Helminen, H. J. Long-distance running causes site-dependent decrease of cartilage glycosaminoglycan content in the knee joints of beagle dogs. Arthritis Rheum. 36, 1451–1459 (1993).
Fang, C. et al. Tissue distribution and measurement of cartilage oligomeric matrix protein in patients with magnetic resonance imaging-detected bone bruises after acute anterior cruciate ligament tears. J. Orthop. Res. 19, 634–641 (2001).
Arokoski, J. P. et al. Decreased birefringence of the superficial zone collagen network in the canine knee (stifle) articular cartilage after long distance running training, detected by quantitative polarised light microscopy. Ann. Rheum. Dis. 55, 253–264 (1996).
Hardingham, T. & Bayliss, M. Proteoglycans of articular cartilage: changes in aging and in joint disease. Semin. Arthritis Rheum. 20, 12–33 (1990).
Maroudas, A., Bayliss, M. T., Uchitel-Kaushansky, N., Schneiderman, R. & Gilav, E. Aggrecan turnover in human articular cartilage: use of aspartic acid racemization as a marker of molecular age. Arch. Biochem. Biophys. 350, 61–71 (1998).
Saxne, T. & Heinegård, D. Synovial fluid analysis of two groups of proteoglycan epitopes distinguishes early and late cartilage lesions. Arthritis Rheum. 35, 385–390 (1992).
Heathfield, T. F., Önnerfjord, P., Dahlberg, L. & Heinegård, D. Cleavage of fibromodulin in cartilage explants involves removal of the N-terminal tyrosine sulfate-rich region by proteolysis at a site that is sensitive to matrix metalloproteinase-13. J. Biol. Chem. 279, 6286–6295 (2004).
Cs-Szabo, G., Roughley, P. J., Plaas, A. H. & Glant, T. T. Large and small proteoglycans of osteoarthritic and rheumatoid articular cartilage. Arthritis Rheum. 38, 660–668 (1995).
Melrose, J. et al. Fragmentation of decorin, biglycan, lumican and keratocan is elevated in degenerate human meniscus, knee and hip articular cartilages compared with age-matched macroscopically normal and control tissues. Arthritis Res. Ther. 10, R79 (2008).
Dickinson, S. C. et al. Cleavage of cartilage oligomeric matrix protein (thrombospondin-5) by matrix metalloproteinases and a disintegrin and metalloproteinase with thrombospondin motifs. Matrix Biol. 22, 267–278 (2003).
Arai, K. et al. Analysis of cartilage oligomeric matrix protein (COMP) degradation and synthesis in equine joint disease. Equine Vet. J. 37, 31–36 (2005).
Kojima, T. et al. Early degradation of type IX and type II collagen with the onset of experimental inflammatory arthritis. Arthritis Rheum. 44, 120–127 (2001).
Danfelter, M., Önnerfjord, P. & Heinegård, D. Fragmentation of proteins in cartilage treated with interleukin-1: specific cleavage of type IX collagen by matrix metalloproteinase 13 releases the NC4 domain. J. Biol. Chem. 282, 36933–36941 (2007).
Budde, B. et al. Altered integration of matrilin-3 into cartilage extracellular matrix in the absence of collagen IX. Mol. Cell. Biol. 25, 10465–10478 (2005).
Tillgren, V., Önnerfjord, P., Haglund, L. & Heinegård, D. The tyrosine sulfate-rich domains of the LRR proteins fibromodulin and osteoadherin bind motifs of basic clusters in a variety of heparin-binding proteins, including bioactive factors. J. Biol. Chem. 284, 28543–28553 (2009).
Vaughan, L. et al. D-periodic distribution of collagen type IX along cartilage fibrils. J. Cell Biol. 106, 991–997 (1988).
Eyre, D. R., Pietka, T., Weis, M. A. & Wu, J. J. Covalent cross-linking of the NC1 domain of collagen type IX to collagen type II in cartilage. J. Biol. Chem. 279, 2568–2574 (2004).
Little, C. B. et al. Matrix metalloproteinase 13-deficient mice are resistant to osteoarthritic cartilage erosion but not chondrocyte hypertrophy or osteophyte development. Arthritis Rheum. 60, 3723–3733 (2009).
Goldberg, R. et al. Time dependent release of matrix components from bovine cartilage after IL-1 treatment and the relative inhibition by matrix metalloproteinase inhibitors. Trans. Orthop. Res. Soc. USA 20, 125 (1995).
Halasz, K., Kassner, A., Mörgelin, M. & Heinegård, D. COMP acts as a catalyst in collagen fibrillogenesis. J. Biol. Chem. 282, 31166–31173 (2007).
Thur, J. et al. Mutations in cartilage oligomeric matrix protein causing pseudoachondroplasia and multiple epiphyseal dysplasia affect binding of calcium and collagen I, II, and IX. J. Biol. Chem. 276, 6083–6092 (2001).
Shen, Z., Heinegård, D. & Sommarin, Y. Distribution and expression of cartilage oligomeric matrix protein and bone sialoprotein show marked changes during rat femoral head development. Matrix Biol. 14, 773–781 (1995).
Heinegård, D. Proteoglycans and more—from molecules to biology. Int. J. Exp. Pathol. 90, 575–586 (2009).
Burstein, D., Gray, M., Mosher, T. & Dardzinski, B. Measures of molecular composition and structure in osteoarthritis. Radiol. Clin. North Am. 47, 675–686 (2009).
Gray, M. L. Toward imaging biomarkers for glycosaminoglycans. J. Bone Joint Surg. Am. 91, 44–49 (2009).
Owman, H., Tiderius, C. J., Neuman, P., Nyquist, F. & Dahlberg, L. E. Association between findings on delayed gadolinium-enhanced magnetic resonance imaging of cartilage and future knee osteoarthritis. Arthritis Rheum. 58, 1727–1730 (2008).
Welsch, G. H. et al. In vivo biochemical 7.0 Tesla magnetic resonance: preliminary results of dGEMRIC, zonal T2, and T2* mapping of articular cartilage. Invest. Radiol. 43, 619–626 (2008).
Di Cesare, P. E. et al. Increased degradation and altered tissue distribution of cartilage oligomeric matrix protein in human rheumatoid and osteoarthritic cartilage. J. Orthop. Res. 14, 946–955 (1996).
Poole, A. R. et al. Contents and distributions of the proteoglycans decorin and biglycan in normal and osteoarthritic human articular cartilage. J. Orthop. Res. 14, 681–689 (1996).
Rolauffs, B. et al. Vulnerability of the superficial zone of immature articular cartilage to compressive injury. Arthritis Rheum. 62, 3016–3027 (2010).
Mizrahi, J., Maroudas, A., Lanir, Y., Ziv, I. & Webber, T. J. The “instantaneous” deformation of cartilage: effects of collagen fiber orientation and osmotic stress. Biorheology 23, 311–330 (1986).
Arkill, K. P. & Winlove, C. P. Solute transport in the deep and calcified zones of articular cartilage. Osteoarthritis Cartilage 16, 708–714 (2008).
von Steyern, F. V. et al. Giant-cell tumour of the knee: the condition of the cartilage after treatment by curettage and cementing. J. Bone Joint Surg. Br. 89, 361–365 (2007).
Acknowledgements
The authors gratefully acknowledge grant support from the National Institute of Arthritis and Musculoskeletal and Skin Disorders (NIAMS), the National Institutes of Health of the USA (NIH) and the Swedish Research Council. The immunostained images of COMP distribution were prepared by Karen King.
Author information
Authors and Affiliations
Contributions
D. Heinegård and T. Saxne contributed equally to all aspects of the preparation of this manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Heinegård, D., Saxne, T. The role of the cartilage matrix in osteoarthritis. Nat Rev Rheumatol 7, 50–56 (2011). https://doi.org/10.1038/nrrheum.2010.198
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrrheum.2010.198
This article is cited by
-
Comparison of the medial midline and the anterolateral portal in ankle arthroscopy for the treatment of osteochondral lesions of the medial talus
International Orthopaedics (2024)
-
Miniimplantate bei fokalen chondralen und osteochondralen Defekten am Femur
Knie Journal (2024)
-
Identification of osteoarthritis-characteristic genes and immunological micro-environment features through bioinformatics and machine learning-based approaches
BMC Medical Genomics (2023)
-
Inhibition of fibroblast activation protein ameliorates cartilage matrix degradation and osteoarthritis progression
Bone Research (2023)
-
Authentication of a novel antibody to zebrafish collagen type XI alpha 1 chain (Col11a1a)
BMC Research Notes (2021)