Abstract
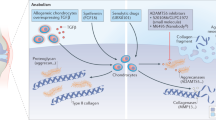
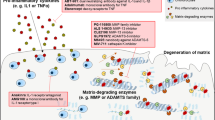
New treatment options are needed for osteoarthritis (OA) to slow down the structural progression of the disease; current therapies mostly target pain and function with minimal effectiveness. OA results from an imbalance between catabolic and anabolic factors, and biologic agents either target specific catabolic proinflammatory mediators, such as cytokines, nitric oxide synthesis, or affect anabolism more generally. Biologic agents have dramatic effects in other rheumatic inflammatory diseases such as rheumatoid arthritis; they were hoped to have similar effects in the treatment of OA. In this Review, we will discuss the three main types of cytokine blockers used in knee and hand OA, which target β-nerve growth factor (β-NGF), IL-1β or TNF. We will also discuss inhibitors of nitrogen oxide production and the use of growth factors to treat OA. Among the targeted agents, anti-β-NGF therapy has shown promising results, although cases of rapid destructive arthropathy caution against its widespread use. The future of therapies targeting cytokines, nitrogen oxide synthesis and growth factors in OA is questionable, as results from clinical trials have been repeatedly negative. Strategies in OA therapy need to be reconsidered. New molecules emerging from preclinical data should focus on treating the early phase of the disease where damage may be reversible, and treatment should be modified to fit each patient.
Key Points
-
Targeted therapy against β-nerve growth factor (β-NGF) in knee osteoarthritis (OA) has resulted in dramatic improvements in symptoms but reports of unexpected, rapid, destructive arthropathies suggest major safety concerns
-
Anti-IL-1β therapy using either intra-articular injection or systemic administration failed to demonstrate any clinical improvement in patients with knee OA
-
Systemic and subcutaneous injections have been used to deliver anti-TNF therapy in patients with polyarticular hand OA and knee OA, respectively; neither strategy has resulted in structural effects or clinical improvement
-
Therapies targeting nitrogen oxide synthesis (administered orally) or local delivery of growth factors in patients with knee OA did not show clinical or structural improvements
-
Future strategies should differentiate between those agents aiming to reduce pain, such as anti-β-NGF treatments, and those targeting structural evolution, which have had disappointing results
-
Emerging therapies should fit the natural progression of OA, focus on early disease where changes might be reversible, and take into account the location and heterogeneity of the disease
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Felson, D. T. et al. Osteoarthritis: new insight. Part 1: The disease and its risk factors. Ann. Intern. Med. 133, 635–646 (2000).
Bijlsma, J. W., Berenbaum, F. & Lafeber, F. P. Osteoarthritis: an update with relevance for clinical practice. Lancet 377, 2115–2126 (2011).
Felson, D. Epidemiology of osteoarthritis. In Osteoarthritis (eds Brandt, K. D., Doherty, M. & Lohmander, L. S.) 9–16 (Oxford University Press, 2003).
Pottie, P. et al. Obesity and osteoarthritis: more complex than predicted! Ann. Rheum. Dis. 65, 1403–1405 (2006).
Zhang, W. et al. OARSI recommendations for the management of hip and knee osteoarthritis, Part II: OARSI evidence-based, expert consensus guidelines. Osteoarthritis Cartilage 16, 137–162 (2008).
Zhang, W. et al. EULAR evidence based recommendations for the management of hand osteoarthritis: report of a Task Force of the EULAR Standing Committee for International Clinical Studies Including Therapeutics (ESCISIT). Ann. Rheum. Dis. 66, 377–388 (2007).
Hochberg, M. C. et al. American College of Rheumatology 2012 recommendations for the use of non pharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res. (Hoboken) 64, 455–474 (2012).
Cooper, C. et al. Efficacy and safety of oral strontium ranelate for the treatment of knee osteoarthritis: rationale and design of randomised, double-blind, placebo-controlled trial. Curr. Med. Res. Opin. 28, 231–239 (2012).
Sellam, J. & Berenbaum, F. The role of synovitis in pathophysiology and clinical symptoms of osteoarthritis. Nat. Rev. Rheumatol. 6, 625–635 (2010).
Mapp., P. I. & Walsh, D. A. Mechanisms and targets of angiogenesis and nerve growth in osteoarthritis. Nat. Rev. Rheumatol. 8, 390–398 (2012).
Pelletier, J. P., Martel-Pelletier, J. & Abramson, S. E. Osteoarthritis, an inflammatory disease: potential implication for the selection of new therapeutic targets. Arthritis Rheum. 44, 1237–1247 (2001).
Chevalier, X. Physiopathogenesis of osteoarthritis. The osteoarthritic cartilage. Presse Med. 27, 81–87 (1998).
Goldring, S. R., and Goldring, M. B. The role of cytokines in cartilage matrix degeneration in osteoarthritis. Clin. Orthop. Rel. Res. 427, S27–S36 (2004).
Chevalier, X. Upregulation of enzymatic activity by interleukin-1 in osteoarthritis. Biomed. Pharmacother. 51, 58–62 (1997).
Benito, M. J., Veale, D. J., Fitzgerald, O., Van den Berg, W. B. & Bresnisham, B. Synovial tissue inflammation in early and late osteoarthritis. Ann. Rheum. Dis. 64, 1263–1267 (2005).
Blom, A. B. et al. Crucial role of macrophages in matrix metalloproteinases-mediates cartilage destruction during experimental osteoarthritis. Arthritis Rheum. 56, 147–157 (2007).
Bondeson, J. Activated synovial macrophages as target for osteoarthritis. Curr. Drug Targets 11, 576–585 (2010).
Hill, C. L. et al. Synovitis detected on magnetic resonance imaging and its relation to pain and cartilage loss in knee osteoarthritis. Ann. Rheum. Dis. 66, 1599–1603 (2007).
Menashe, L. et al. The diagnostic performance of MRI in osteoarthritis: a systematic review and meta-analysis. Osteoarthritis Cartilage 20, 13–21 (2012).
Ayral, X., Pickering, E. H., Woodworth, T. G., Mackillop, N. & Dougados, M. Synovitis: a potential predictive factor of structural progression of medial tibiofemoral knee osteoarthritis—results of a 1 year longitudinal arthroscopic study in 422 patients. Osteoarthritis Cartilage 13, 361–367 (2005).
Bonnet, C. S. & Walsh, D. A. Osteoarthritis, angiogenesis and inflammation. Rheumatology (Oxford) 44, 7–16 (2005).
Pesesse, L., Sanchez, C. & Henrotin, Y. Osteochondral plate angiogenesis: a new treatment target in osteoarthritis. Joint Bone Spine 78, 144–149 (2011).
Sanchez, C. et al. Osteoblasts from the sclerotic subchondral bone downregulate aggrecan but upregulate metalloproteinases expression by chondrocytes. This effect is mimicked by interleukin-6, interleukin–1β and oncostatin M pre-treated non-sclerotic osteoblasts. Osteoarthritis Cartilage 13, 979–987 (2005).
Mamelud, C. J. Cytokines as therapeutic targets for osteoarthritis. Biodrugs 18, 23–35 (2004).
Sachs, D. et al. Tumour necrosis factor-alpha, interleukin–1β and interleukin-8 induce persistent mechanical nociceptor hypersensitivity. Pain 96, 89–97 (2002).
Blom, A. B., van der Kraan, P. M. & van der Berg, W. B. Cytokine targeting in osteoarthritis. Curr. Drug Targets 8, 283–292 (2007).
Abramson, S. B. & Yazici, Y. Biologics in the development for rheumatoid arthritis: relevance to osteoarthritis. Adv. Drug Del. Rev. 58, 212–215 (2006).
Chevalier, X. Intraarticular treatments for osteoarthritis: new perspectives. Curr. Drug Targets 11, 546–560 (2010).
Wood, J. N. Nerve growth factor and pain. N. Engl. J. Med. 363, 1572–1573 (2010).
Isola, M. et al. Nerve growth factor concentrations in the synovial fluid from healthy dogs and dogs with secondary osteoarthritis. Vet. Comp. Orthop. Traumatol. 24, 279–284 (2011).
Barthel, C. et al. Nerve growth factor and receptor expression in rheumatoid arthritis and spondyloarthritis. Arthritis Res. Ther. 11, R82 (2009).
Raychaudhuri, S. P., Raychaudhuri, S. K., Atkuri, K. R., Herzenberg, L. A. & Herzenberg, L. A. Nerve growth factor: A key local regulator in the pathogenesis of inflammatory arthritis. Arthritis Rheum. 63, 3243–3252 (2011).
Kumar, V., and Mahal, B. A. NGF—the TrkA to successful pain treatment. J. Pain Res. 5, 279–287 (2012).
Brown, M. T. et al. Tanezumab reduces osteoarthritic knee pain: results of a randomized, double-blind, placebo-controlled phase III trial. J. Pain 13, 790–798 (2012).
Nagashima, H., Suzuki, M., Araki, S., Yamabe, T. & Muto, C. Tanezumab Investigators. Preliminary assessment of the safety and efficacy of tanezumab in Japanese patients with moderate to severe osteoarthritis of the knee: a randomized, double-blind, dose-escalation, placebo-controlled study. Osteoarthritis Cartilage 19, 1405–1412 (2011).
Schnitzer, T. J. et al. Long-term open-label study of tanezumab for moderate to severe osteoarthritic knee pain. Osteoarthritis Cartilage 19, 639–646 (2011).
Lane, N. E. et al. Tanezumab for the treatment of pain from osteoarthritis of the knee. N. Engl. J. Med. 363, 1521–1531 (2010).
Katz, N. et al. Efficacy and safety of tanezumab in the treatment of chronic low back pain. Pain 152, 2248–2258 (2011).
Chevalier, X., Mugnier, B. & Bouvenot, G. Targeted anti-cytokine therapies for osteoarthritis. Bull. Acad. Natl Med. 190, 1411–1420 (2006).
Seidel, M. F. & Lane, N. E. Control of arthritis pain with anti-nerve-growth factor: Risk and benefit. Curr. Rheumatol. Rep. 6, 583–588 (2012).
Chandrasekhar, S., Harvey, A. K. & Hrubey, P. S. Intra-articular administration of interleukin-1 causes prolonged suppression of cartilage proteoglycan synthesis in rats. Matrix 12, 1–10 (1992).
Caron, J. P. et al. Chondroprotective effect of intra-articular injections of interleukin-1 receptor antagonist in experimental osteoarthritis. Suppression of collagenase-1 expression. Arthritis Rheum. 39, 1535–1544 (1996).
Pelletier, J. P. et al. In vivo suppression of early experimental osteoarthritis by interleukin-1 receptor antagonist using gene therapy. Arthritis Rheum. 40, 1012–1019 (1997).
Fernandes, J. C. et al. In vivo transfer of interleukin-1 receptor antagonist gene in osteoarthritic rabbit knee joints: prevention of osteoarthrosis progression. Am. J. Pathol. 154, 1159–1169 (1999).
Zhang, X, Mao, Z. & Yu, C. Suppression of early experimental osteoarthritis by gene transfer of interleukin-1 antagonist and interleukin-10. J. Orthop. Res. 22, 742–750 (2004).
Wang, H.J. et al. Suppression of experimental osteoarthritis by adenovirus-mediated double gene transfer. Chin. Med. J. 119, 1365–1373 (2006).
Santangelo, K. S., Nuovo, G. J. & Bertone, A. L. In vivo reduction or blockade of interleukin-1β in primary osteoarthritis influences expression of mediators implicated in pathogenesis. Osteoarthritis Cartilage 20, 1610–1618 (2012).
Clements, K. M. et al. Gene deletion of either interleukin-1β, interleukin-1β converting enzyme, inducible nitric oxide synthase, or stromelysin 1 accelerates the development of knee osteoarthritis in mice after surgical transection of the medial collateral ligament and partial medial menisectomy. Arthritis Rheum. 48, 3352–3363 (2003).
Bougault, C. et al. Stress-induced cartilage degradation does not depend on NLRP3 inflammasome in osteoarthritis. Arthritis Rheum. 64, 3972–3981 (2012).
Chevalier, X. et al. Safety study of intraarticular injection of interleukin 1 receptor antagonist in patients with painful knee osteoarthritis: a multicenter study. J. Rheumatol. 32, 1317–1323 (2005).
Chevalier, X. et al. Intraarticular injection of anakinra in osteoarthritis of the knee: a multicenter, randomized, double-blind, placebo-controlled study. Arthritis Rheum. 61, 344–352 (2009).
Loeuille, D. et al. Macroscopic and microscopic features of synovial membrane inflammation in the osteoarthritic knee: Correlating magnetic resonance imaging findings with disease severity. Arthritis Rheum. 52, 3492–3501 (2005).
Cohen, S. et al. A randomized, double-blind study of AMG 108 (a fully human monoclonal antibody to IL-1R1) in patients with osteoarthritis of the knee. Arthritis Res. Ther. 13, R125 (2011).
Chevalier, X., Conrozier, T. H. & Richette, P. Desperately looking for the right target in osteoarthritis—The anti IL-1 strategy. Arthritis Res. Ther. 13, 124 (2011).
Hanna, F. S. et al. High sensitivity C-reactive protein is associated with lower tibial cartilage volume but not lower patella cartilage volume in healthy women at midlife. Arthritis Res. Ther. 10, R27 (2008).
Sharif, M., Shepstone, L., Elson, C. J., Dieppe, P. A. & Kirwan, J. R. Increased serum C reactive protein may reflect events that precede radiographic progression in osteoarthritis of the knee. Ann. Rheum. Dis. 59, 71–4 (2000).
Bacconnier, L., Jorgensen, C. & Fabre, S. Erosive osteoarthritis of the hand: clinical experience with anakinra. Ann. Rheum. Dis. 68, 1078–1079 (2009).
Bigoni, M. et al. Acute and late changes in intraarticular cytokine levels following anterior cruciate ligament injury. J. Orthop. Res. 31, 315–321 (2013).
Brown, C., Toth, A. & Magnussen, R. Clinical benefits of intra-articular anakinra for persistent knee effusion. J. Knee Surg. 24, 61–65 (2011).
Kraus, V. B. et al. Effects of intraarticular IL1-Ra for acute anterior cruciate ligament knee injury: a randomized controlled pilot trial (NCT00332254). Osteoarthritis Cartilage 20, 271–278 (2012).
Stannus, O. et al. Circulating levels of IL-6 and TNF-α are associated with knee radiographic osteoarthritis and knee cartilage loss in older adults. Osteoarthritis Cartilage 18, 1441–1447 (2010).
Malfait, A. M. et al. Intra-articular injection of tumor necrosis factor-α in the rat: an acute and reversible in vivo model of cartilage proteoglycan degradation. Osteoarthritis Cartilage 17, 627–635 (2009).
Zhang, Q., Lv, H., Chen, A., Liu, F. & Wu, X. Efficacy of infliximab in a rabbit model of osteoarthritis. Connect. Tissue Res. 53, 355–358 (2012).
Punzi, L., Ramonda, R. & Sfriso, P. Erosive osteoarthritis. Best Pract. Res. Clin. Rheumatol. 18, 739–758 (2004).
Punzi, L. et al. Value of C reactive protein in the assessment of erosive osteoarthritis of the hand. Ann. Rheum. Dis. 64, 955–957 (2005).
Dziedzic, K. S. Osteoarthritis: best evidence for best therapies in hand osteoarthritis. Nat. Rev. Rheumatol. 7, 258–260 (2011).
Michon, M, Maheu, E. & Berenbaum, F. Assessing health-related quality of life in hand osteoarthritis: a literature review. Ann. Rheum. Dis. 70, 921–928 (2011).
Hill, S., Dziedzic, K. S. & Nio Ong, B. Patients' perceptions of the treatment and management of hand osteoarthritis: a focus group enquiry. Disabil. Rehabil. 33, 1866–1872 (2011).
Avouac, J., Marini-Portugal, A. & Chevalier, X. A propos d'un cas d'arthrose digitale érosive: réponse spectaculaire aux anti-TNF α. Revue Rhum. 71, 158 (2004).
Magnano, M. D., Chakravarty, E. F. & Broudy, C. A pilot study of tumor necrosis factor inhibition in erosive/inflammatory osteoarthritis of the hands. J. Rheumatol. 34, 1323–1327 (2007).
Güler-Yüksel . et al. Treatment with TNF-α inhibitor infliximab might reduce hand osteoarthritis in patients with rheumatoid arthritis. Osteoarthritis Cartilage 18, 1256–1262 (2010).
Verbruggen, G., Wittoek, R., Cruyssen, B. V. & Elewaut, D. Tumour necrosis factor blockade for the treatment of erosive osteoarthritis of the interphalangeal finger joints: a double blind, randomised trial on structure modification. Ann. Rheum. Dis. 71, 891–898 (2012).
Chevalier, X. et al. A randomized, multicentre, double blind, placebo-controlled trial of anti TNF α (adalimumab) in refractory hand osteoarthritis: the Dora study. Arthritis Rheum. 64, 10 Suppl 10: 2472 (2012).
Fioravanti, A., Fabbroni, M., Cerase, A. & Galeazzi, M. Treatment of erosive osteoarthritis of the hands by intra-articular infliximab injections: a pilot study. Rheumatol. Int. 29, 961–965 (2009).
Grunke, M. & Schulze-Koops, H. Successful treatment of inflammatory knee osteoarthritis with tumour necrosis factor blockade. Ann. Rheum. Dis. 65, 555–556 (2006).
Bollet, A. J. Edema of the bone marrow can cause pain in osteoarthritis and other diseases of bone and joints. Ann. Intern. Med. 134, 591–593 (2001).
Hayes, C. W. et al. Osteoarthritis of the knee: comparison of MR imaging findings with radiographic severity measurements and pain in middle-aged women. Radiology 237, 998–1007 (2005).
Schue, J. R. et al. Treatment of knee osteoarthritis with intraarticular infliximab. Arthritis Rheum. 63 (Suppl 9), S325, 826 (2011).
Maksymowych, W. P. et al. Targeting tumor necrosis factor alleviates signs and symptoms of inflammatory osteoarthritis. Arthritis Res. Ther. 14, R206 (2012).
Abramson, S. B. et al. The role of nitric oxide in tissue destruction. Best Pract. Res. Clin. Rheumatol. 15, 831–845 (2001).
Pelletier, J. P. et al. Selective inhibition of inducible nitric oxide synthase reduces progression of experimental osteoarthritis in vivo: possible link with the reduction in chondrocyte apoptosis and caspase 3 level. Arthritis Rheum. 43, 1290–1299 (2000).
Hellio le Graverand, M. P. et al. A 2-year randomised, double-blind, placebo-controlled, multicentre study of oral selective iNOS inhibitor, cindunistat (SD-6010), in patients with symptomatic osteoarthritis of the knee. Ann. Rheum. Dis. 72, 187–195 (2013).
Cook, S. D. & Rueger, D. C. Osteogenic protein-1: biology and applications. Clin. Orthop. Relat. Res. 324, 29–38 (1996).
Sieber, C., Kopf, J., Hiepen, C. & Knaus, P. Recent advances in BMP receptor signaling. Cytokine Growth Factor Rev. 20, 343–355 (2009).
Wozney, J. M. Overview of bone morphogenetic proteins. Spine (Phila. Pa. 1976) 27 (16 Suppl. 1), S2–S8 (2002).
Hunter, D. J. et al. Phase 1 safety and tolerability study of BMP-7 in symptomatic knee osteoarthritis. BMC Musculoskelet. Disord. 11, 232 (2010).
Mc Pherson, R., Flechenshar, K., Hellot, S. & Eckstein, F. A randomized, double–blind, placebo controlled, multicenter study of FGF 18 administered intra articularly using single or multiple ascending doses in patients with primary knee osteoarthritis (0A), not expected to require knee surgery within a year. Osteoarthritis Cartilage 19 (Suppl. 1), S35–S36 (2011).
Stewart, K. et al. The effect of growth factor treatment on meniscal chondrocyte proliferation and differentiation on polyglycolic acid scaffolds. Tissue Eng. 13, 271–280 (2007).
Ellsworth, J. L. et al. Fibroblast growth factor-18 is a trophic factor for mature chondrocytes and their progenitors. Osteoarthitis Cartilage 10, 308–320 (2002).
Ohbayashi, N. et al. FGF18 is required for normal cell proliferation and differentiation during osteogenesis and chondrogenesis. Genes Dev. 16, 870–879 (2002).
Moore, E. E. et al. Fibroblast growth factor-18 stimulates chondrogenesis and cartilage repair in a rat model of injury-induced osteoarthritis. Osteoarthritis Cartilage 13, 623–631 (2005).
van der Kraan, P. M. & van den Berg, W. B. Osteophytes: relevance and biology. Osteoarthritis Cartilage 15, 237–244 (2007).
Richette, P. et al. A high interleukin 1 receptor antagonist/IL-1β ratio occurs naturally in knee osteoarthritis. J. Rheumatol. 35, 1650–1654 (2008).
Hunter, D. J. Pharmacologic therapy for osteoarthritis—the era of disease modification. Nat. Rev. Rheumatol. 7, 13–22 (2011).
Scanzello, C. R. & Goldring, SR. The role of synovitis in osteoarthritis pathogenesis. Bone 51, 249–257 (2012).
Scanzello, C. R. et al. Synovial inflammation in patients undergoing arthroscopic meniscectomy: molecular characterization and relationship to symptoms. Arthritis Rheum. 63, 391–400 (2011).
Bondeson, J. et al. The role of synovial macrophages and macrophage-produced mediators in driving inflammatory and destructive responses in osteoarthritis. Arthritis Rheum. 62, 647–657 (2010).
Bondeson, J. Are we moving in the right direction with osteoarthritis drug discovery? Expert Opin. Ther. Targets 15, 1355–1368 (2011).
Pelletier, J. P. & Martel-Pelletier, J. DMOAD developments: present and future. Bull. NYU Hosp. Jt Dis. 65, 242–248 (2007).
Genovese, M. C. et al. Combination therapy with etanercept and anakinra in the treatment of patients with rheumatoid arthritis who have been treated unsuccessfully with methotrexate. Arthritis Rheum. 50, 1412–1419 (2004).
Rose-John, S., Waetzig, G. H., Scheller, J., Grötzinger, J. & Seegert, D. The IL-6/sIL-6R complex as a novel target for therapeutic approaches. Expert Opin. Ther. Targets 11, 613–24 (2007).
Desgeorges, A. et al. Concentrations and origins of soluble interleukin-6 receptor-α in serum and synovial fluid. J. Rheumatol. 24, 1510–1516 (1997).
Blom, A. B., van Lent, P. L., van der Kraan, P. M. & van den Berg, W. B. To seek shelter from the WNT in osteoarthritis? WNT-signaling as a target for osteoarthritis therapy. Curr. Drug Targets 11, 620–629 (2010).
Tanaka, Y. & Yamaoka, K. JAK inhibitor tofacitinib for treating rheumatoid arthritis: from basic to clinical. Mod. Rheumatol. http://dx.doi.org/10.1007/s10165-012-0799-2.
Author information
Authors and Affiliations
Contributions
X. Chevalier researched data for the article and wrote the article. All authors contributed substantially to discussion of content, and reviewing and editing the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Figure 1
MRI of a knee with OA. (PDF 421 kb)
Supplementary Figure 2
MRI showing a reduction in the extent of bone marrow oedema 6 months after repeated subcutaneous adalimumab injections in a patient with knee OA. (PDF 457 kb)
Rights and permissions
About this article
Cite this article
Chevalier, X., Eymard, F. & Richette, P. Biologic agents in osteoarthritis: hopes and disappointments. Nat Rev Rheumatol 9, 400–410 (2013). https://doi.org/10.1038/nrrheum.2013.44
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrrheum.2013.44
This article is cited by
-
Calycosin ameliorates osteoarthritis by regulating the imbalance between chondrocyte synthesis and catabolism
BMC Complementary Medicine and Therapies (2024)
-
Role of circular RNAs in osteoarthritis: update on pathogenesis and therapeutics
Molecular Genetics and Genomics (2023)
-
Synovial fibroblast-miR-214-3p-derived exosomes inhibit inflammation and degeneration of cartilage tissues of osteoarthritis rats
Molecular and Cellular Biochemistry (2023)
-
Synovial inflammation in osteoarthritis progression
Nature Reviews Rheumatology (2022)
-
Current understanding of osteoarthritis pathogenesis and relevant new approaches
Bone Research (2022)