Article Text
Abstract
Background Elective switching between anti-tumour necrosis factor (TNF) agents not necessarily dictated by efficacy or tolerability occurs in clinical practice. A study was undertaken to evaluate prospectively the impact of elective switching of patients with Crohn's disease well controlled with intravenous infliximab to subcutaneous adalimumab in a controlled trial.
Methods An open-label randomised single-centre trial recruited 73 patients with ongoing response to at least 6 months of scheduled maintenance infliximab. Patients were randomised to continue intravenous 5 mg/kg infliximab or to switch to subcutaneous adalimumab 80 mg at baseline followed by 40 mg every other week for 1 year. Dose optimisation was allowed for intermittent flares, and patients with loss of response or intolerance could cross over to the alternative treatment group. Tolerability, patient preference and efficacy of both treatment options were the primary outcomes.
Results Dose optimisation or interruption of treatment occurred in 17/36 patients (47%) in the adalimumab group and in 6/37 patients (16%) in the infliximab group (p=0.006). One patient interrupted infliximab treatment and 10 patients interrupted adalimumab treatment (p=0.003), mostly for loss of tolerance. Overall, patients preferred adalimumab treatment. All five serious adverse events were related to complicated Crohn's disease and occurred in patients randomised to adalimumab. Injection site reactions were more frequent than infusion reactions (8 vs 1, p=0.01), but only the latter caused cessation of further dosing. Anti-TNF serum levels were stable throughout the 1-year period in both groups.
Conclusion Elective switching from infliximab to adalimumab is associated with loss of tolerance and loss of efficacy within 1 year. Adherence to the first anti-TNF agent is recommended.
- Crohn's disease
- infliximab
- adalimumab
- anti TNF antibodies
- medical therapy
- pharmacokinetics
- Gastrointestinal muscle
- IBD
- IBD models
- IBD clinical
- IBD basic research
- inflammatory mechanisms
- hepatorenal syndrome
Statistics from Altmetric.com
- Crohn's disease
- infliximab
- adalimumab
- anti TNF antibodies
- medical therapy
- pharmacokinetics
- Gastrointestinal muscle
- IBD
- IBD models
- IBD clinical
- IBD basic research
- inflammatory mechanisms
- hepatorenal syndrome
Significance of this study
What is already known about this subject?
Anti-TNF agents given as maintenance therapy are effective in controlling Crohn's disease.
Both subcutaneous adalimumab and intravenous infliximab are available.
Elective switching for reasons of convenience has entered clinical practice.
What are the new findings?
Elective switching to adalimumab leads to worse outcomes than maintaining treatment with infliximab.
Intolerance was a more common reason than loss of efficacy for stopping adalimumab.
Both strategies result in durable serum levels.
How might it impact on clinical practice in the foreseeable future?
Adherence to infliximab in patients with a durable response is a better choice than elective switching to adalimumab.
Introduction
The efficacy of adalimumab (ADA) and infliximab (IFX), two monoclonal IgG1 anti-tumour necrosis factor (TNF) antibodies, as maintenance treatment of refractory luminal Crohn's disease has been well established.1–3 Both agents are also used to control rheumatoid arthritis, spondyloarthropathy and other inflammatory disorders. Although the mechanism of action of anti-TNF agents is not fully understood, both IFX and ADA have been shown to induce apoptosis of activated lymphocytes, to heal mucosal ulcers in the colon, to reduce hospitalisations and to spare corticosteroids.4–8 Maintenance therapy with anti-TNF agents has become standard of care since it improves long-term disease control, prevents symptom flares and reduces immunogenicity. Prospective clinical trials comparing the efficacy of ADA and IFX in Crohn's disease or in other indications are not currently available. The choice between the two agents is therefore most often based on patient preference or other arguments beyond clinical efficacy. The fully human antibody ADA is administered subcutaneously every other week, whereas the chimeric antibody IFX is given less frequently but in hospital or at an infusion clinic. Since ADA can be self-administered at home, switching from IFX to ADA in patients well controlled with maintenance therapy for reasons of convenience or to suit hospital budgets has been increasingly observed in clinical practice. A prospective controlled trial investigating the impact of such a strategy on long-term disease control and on tolerability has not been performed. For patients with rheumatoid arthritis, a wider range of anti-TNF treatment options has been available for many years. However, even in this indication, no prospective controlled data on switching between agents in patients with a durable response to the first agent are available.
Elective switching from IFX to ADA has entered clinical practice in patients with Crohn's disease to avoid intravenous administration. As there are no prospective trial data, we designed a randomised open-label single-centre 1-year study to investigate this issue in a real-life clinical setting.
Methods
Patients
Men and women age ≥18 years with luminal Crohn's disease treated with scheduled IFX maintenance therapy started at least 6 months before without episodic use during that time period were eligible for the study. A durable complete clinical response with stable IFX dosing intervals of at least 6 weeks for the last 6 months was required. Complete response was defined by physician global assessment of signs and symptoms, but the Crohn's Disease Activity Index (CDAI9) at baseline had to be <200. Patients with a draining abdominal enterocutaneous fistula, with a medical condition or laboratory tests precluding further anti-TNF therapy, with previous exposure to ADA, receiving IFX doses >5 mg/kg intravenously and those with an imminent need for surgery were excluded. After written informed consent, patients were randomly allocated either to continue IFX 5 mg/kg intravenously at the same interval for 56 weeks or to switch to ADA. Patients in the ADA group received 80 mg subcutaneously at inclusion and 40 mg subcutaneously every other week for 54 weeks (figure 1). Random allocation was based on a centrally stored randomly generated list that was not accessible to the investigators. Patients with a disease flare were allowed dose intensification. The prespecified anti-TNF dose adjustments were: in the ADA group, step-up to 40 mg every week and, in the IFX group, a decrease of the dosing interval with 2-week decrements. Short (4-week) courses of steroids were also allowed per protocol. Patients with complete loss of response or intolerance were able to cross over to the alternative treatment group. Serum levels of IFX were assessed using an in-house ELISA (cut-off 0.30 μg/ml) and ADA serum levels were measured at Abbott, Ludwigshafen, Germany with a similar ELISA.
Design of the trial. Eligible patients were randomised (1:1) to one of the two study groups. Patients with loss of response were eligible for medical rescue therapy. Crossover to the alternative study group was allowed for intolerance or for definitive loss of response. ADA, adalimumab; EOW, every other week; EW, every week; IFX, infliximab; LOR, loss of response; SC, subcutaneous.
Endpoints and assessments
The main endpoints of this trial were the proportion of patients in the ADA group preferring ADA over IFX and the proportion of patients who needed rescue therapy with short courses of steroids or intensified anti-TNF dosing or who had to stop the assigned anti-TNF agent. The proportion of patients with an injection- or infusion-related reaction and the proportion of patients with an increase in the CDAI of >100 above baseline were additional endpoints. Clinical disease activity was assessed with diary-based CDAI.9 Quality of life was measured with the Inflammatory Bowel Disease Questionnaire (IBDQ).10 As a biomarker of disease activity, C-reactive protein (CRP) was assessed at every visit. For safety liver tests, serum creatinine, electrolytes and a full blood count were obtained. Serum samples were banked at every visit for drug level analysis. Patient preference was questioned in the ADA arm at all study visits. Patients were asked whether they preferred ADA over IFX, IFX over ADA or if they were indifferent. Items in the questionnaire were: general preference, benefit from the therapy, mode of administration, impact on activities of daily life, burden of adverse events and financial implications. The reported preferences were analysed on an intention-to-treat level and calculations were based on the entire cohort of 36 patients. The sum of proportions is therefore not necessarily 100%.
The statistical hypothesis was that patients in the ADA group would prefer this treatment subjectively. We estimated that at least 60% of patients would prefer ADA. With 50 patients in every group, a power of 80% would be reached to test this hypothesis based on a single population binomial test. Differences in proportions were tested with the Fisher exact or χ2 test. Continuous variables were assessed with Mann-Whitney tests. A Data Safety and Monitoring Board (DSMB) comprising a clinical rheumatologist and a clinical immunologist with ample experience in biological therapy convened after 50% of patients had been recruited to the trial.
Results
Patient characteristics
Seventy-three patients were randomised to the two treatment groups (36 ADA, 37 IFX). The demographic characteristics of the patients were evenly distributed among the treatment arms (table 1). A small number of patients were on concomitant immunosuppression (ADA 17%, IFX 5%) and most were on 8-weekly IFX before entering into the trial (83% ADA, 76% IFX). Less than one-third of patients in both groups actively smoked and none of the patients changed their smoking habits while in the trial.
Demographic characteristics of study patients in the two treatment groups
Clinical efficacy
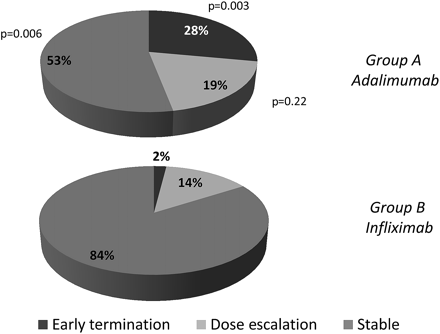
Dose intensification or early treatment termination was observed in 17 patients (47%) in the ADA group and six patients (16%) in the IFX group (p=0.003). Ten of 36 patients stopped ADA therapy due to loss of response or intolerance compared with one of 37 patients in the IFX group (28% vs 2%, p<0.01). The one patient who stopped IFX was successfully treated with ADA and eight of the 10 patients who stopped ADA treatment returned to IFX therapy (figures 2 and 3). This was successful in all eight, but four patients needed IFX dose intensification within 1 year after restarting. The reasons for early treatment termination were loss of tolerance in six of 10 patients on ADA and in the one patient who stopped IFX. Another four patients in the ADA group stopped for loss of efficacy. Refractory eczema with fatigue or arthralgias (n=2), general malaise and diarrhoea following injections (n=2) and fatigue plus inability to comply with injections (n=2) led to ADA intolerance and an infusion reaction to IFX intolerance. The median CDAI at time of early termination in the ADA group was 184 (IQR 44–235) compared with 78 (IQR 35–134, p=0.10) at baseline. Dose intensification occurred in 10 of 36 patients in the ADA group, of whom three stopped ADA for loss of response later, and in five of 37 patients in the IFX group (p=0.2). The median time to dose intensification was 24 weeks (IQR 17–33) in the ADA group and 32 weeks (IQR 16–37) in the IFX arm (p=0.64). After more than 50% of the planned patient population had been recruited, the DSMB analysed the interim data and advised against further recruitment to the trial based on the large difference in discontinuation of the assigned treatment between the two groups.
Flow chart of patient disposition throughout the trial.
Graphical overview of the need for dose adjustments and the proportion of patients discontinuing the assigned treatment throughout the trial.
An increase in CDAI of ≥100 points was observed in seven of 37 patients in the IFX group and in 10 of 36 patients in the ADA group while on the initially assigned treatment. Median IBDQ values at baseline and at week 56 were comparable in both groups and the medians stayed well in the range compatible with disease remission throughout the trial (ADA: week 0: 197 (IQR 181–212), week 54: 193 (IQR 160–214); IFX: week 0: 191 (IQR 172–203), week 54: 188 (IQR 170–204).
Patient preferences
In general, significantly more patients preferred ADA over IFX at most time points in the trial. The results for the individual items were stable over time and were pooled for analysis (figure 4). For most items in the questionnaire, patients preferred ADA over IFX except for the financial impact of the treatment.
Patient-reported preference for the different treatment options. Only patients in the adalimumab group were polled and calculations were based on the entire randomised population. (A) Overall preference at different time points. (B) Specific preference for different items on the questionnaire. All time points were pooled for the latter analysis. ADA, adalimumab; IFX, infliximab.
Adverse events
Overall, 30 of 36 patients in the ADA group (83%, total number of events 50) and 27 of 37 in the IFX group (73%, total number of events 39) reported side effects that were considered to be at least possibly related to the anti-TNF therapy. Upper respiratory tract infections, fatigue, skin lesions and injection site reactions were the most frequently occurring events. Injection site reactions were all mild and occurred in eight of 36 patients in the ADA group and one patient had an infusion reaction in the IFX group (p=0.01), but only this patient became intolerant due to the drug-related reaction. All serious adverse events occurred in five patients originally assigned to the ADA group (box 1, p<0.05 vs IFX group); two patients had returned to IFX when the adverse event occurred.
Serious adverse events
Adalimumab group: continuing adalimumab after serious adverse event (n=1)
Terminal ileitis and secondary ileus: hospitalisation, antibiotics, adalimumab dose escalation.
Adalimumab group: stopping adalimumab after serious adverse event (n=2)
Anal abscess: hospitalisation and drainage, infliximab restarted.
Crohn's disease flare with perforation and ileal abscess: hospitalisation, antibiotics, resection.
Adalimumab group returned to infliximab: continuing infliximab after serious adverse event (n=2)
Anal abscess and stenosis, complex fistula: drainage and dilation, infliximab restarted.
Anastomotic stenosis: hospitalisation and dilation, infliximab restarted.
All patients with a serious adverse event had been randomised to adalimumab, two patients had already returned to infliximab when the serious adverse event occurred.
Serum drug levels
An inter-individual variation in drug levels was observed, but median IFX serum trough levels and ADA serum levels remained stable over time until week 54 (figure 5). Anti-TNF serum levels were undetectable in two patients (5%) at any time point in the two treatment arms. All patients who left the ADA group and returned to IFX treatment had detectable IFX trough levels at the end of follow-up (median 59 (IQR 54–62) weeks from baseline), and the levels were comparable to or higher than baseline. However, the IFX dosing interval of four of eight patients had been shortened at the end of follow-up, with sustained detectable trough levels after dose adjustment.
Serum levels of infliximab and adalimumab in the two treatment groups at different time points throughout the trial. Box plots represent medians and IQR. Error bars range from the 5th to the 95th percentile. Different ELISAs were used to analyse the trough levels of adalimumab and infliximab.
Discussion
Biological treatments, particularly anti-TNF agents, have become a standard treatment option for patients with refractory Crohn's disease. In this trial we focused on the impact of electively switching patients between anti-TNF antibodies since, in the current era of managed care and pharmacoeconomic factors, patients leave successful IFX for home-based ADA therapy. Also, college students studying in schools that are a large distance from their gastroenterologist's surgery often consider switching to a subcutaneous agent. The data from our controlled study show that almost one in three patients who were switched to ADA had to return to IFX within the time frame of 1 year despite a clear patient preference for subcutaneously administered ADA therapy. In rheumatoid disorders, additional subcutaneously administered biological agents are being prescribed, and switching between agents for reasons other than clinical efficacy has also become increasingly prevalent. Biological therapies impact profoundly on hospital budgets in many countries, and across indications physicians have been urged by hospital managers to switch patients to a home-based subcutaneous treatment despite ongoing disease control with an intravenously administered biological agent. Surprisingly, no prospectively controlled trial has investigated the efficacy and safety of this strategy in any inflammatory disorder. An observational study in 19 patients with rheumatoid arthritis followed for 16 weeks suggested that switching from IFX to ADA is effective and well tolerated, but a control group was not included in the study.11 In a recently published cohort of patients with rheumatoid arthritis treated with IFX, 70 of 281 patients (25%) stopped IFX electively and the authors observed that the peak incidence of elective discontinuation occurred around the time when a subcutaneous anti-TNF treatment became available as a novel treatment option.12
In contrast to orally administered small molecule drugs, therapeutic antibodies are characterised by immunogenicity leading to loss of response and drug-induced reactions. Thus, even if most patients in our trial responded again to IFX administration after a median of 16 weeks off the drug and had detectable trough levels, loss of response or intolerance may present later in these patients and would eliminate all currently available biological options. The fact that four out of eight patients needed IFX dose intensification after returning to this treatment reinforces the concern on the long-term impact of elective switching.
Most patients who left the ADA group did so for reasons of intolerance rather than for lack of efficacy. Our trial should therefore not be interpreted to show a superior efficacy of IFX maintenance therapy for Crohn's disease. A specifically powered comparative blinded trial is required to answer this question. Also, patients were selected for a durable response and tolerability to IFX for 6 months or longer. Of interest, all major side effects occurred in patients originally assigned to the group that switched to ADA and were related to worsening activity or disease-related complications.
Anti-TNF drug levels were stable over time in the two groups. At all time points, median ADA levels were higher than IFX levels. However, this difference should be interpreted with caution since different assays were used. Also, due to the biweekly dosing scheme, ADA serum levels cannot be considered real ‘trough’ levels as an injection close to the blood sampling may influence the pharmacokinetics. At any time during the trial, no more than two patients had undetectable ADA serum levels. This excludes lack of adherence to ADA injections as a major cause of loss of response.
Our study has several important limitations. First, we opted for an open-label real-life clinical strategy design to avoid a complex double-dummy set up that would require placebo injections every other week and placebo infusions every 8 weeks. However, we assume that the patients who volunteered for this trial had a positive attitude towards elective switching, and the open-label nature is not sufficient to explain why one-third of patients returned to IFX. Second, we only enquired about treatment preference in patients who switched to ADA for obvious reasons. The results of the patient preferences should therefore be interpreted with caution. Unsurprisingly, those patients who remained in the ADA group generally preferred this treatment over IFX. When the individual items of the questionnaire were analysed, only the financial impact of the anti-TNF therapy was conceived to be similar by the patients.
In conclusion, the results of the first randomised trial of elective switching between anti-TNF agents show that, within 1 year, almost one in three patients returned to IFX therapy after electively switching to ADA to control their Crohn's disease. We acknowledge that in exceptional cases a home-based subcutaneous treatment may be more convenient for special groups of patients, but this should not deprive them from further follow-up by an expert physician. However, we clearly caution against switching from IFX to ADA for reasons other than loss of response or of tolerance. Due to the limited number of approved biological agents, persistence with and optimal use of the first anti-TNF agent is even more important in patients with inflammatory bowel disease than in those with other inflammatory disorders.
Acknowledgments
The authors thank Dr Jan Ceuppens and Dr René Westhovens for their valuable contributions in the Data and Safety Monitoring Board.
References
Footnotes
See Commentary, p 169
Protocol details available at: http://clinicaltrials.gov. Full protocol available from the authors upon request. Trial registration number: U of Leuven S50256/ML 4041.
Funding The design and conduct of the trial, data analysis and manuscript writing was performed independently by the authors. All authors had access to the data and decided to jointly submit the manuscript. Adalimumab serum levels were analysed by Abbott GMBH, Ludwigshafen, Germany. Adalimumab was provided for the patients in this trial by Abbott Belgium.
Competing interests SV: grants/research support (UCB), consultancy (Astra-Zeneca, Ferring, Pfizer), speakers bureau (Schering-Plough, Abbott, Ferring, UCB), advisory committee (Shire, Ferring). PR: research grants, lecture fees, consultant fees (Abbott, Centocor, Schering-Plough, UCB). GVA: speaker fee/research support (Centocor, Schering-Plough, Abbott, UCB). GD'H: speaker fee/research support (Centocor, Schering-Plough, Abbott, UCB). The other authors have no conflicts of interest.
Ethics approval Ethics approval was provided by the University of Leuven ethical committee.
Provenance and peer review Not commissioned; externally peer reviewed.