Article Text
Abstract
Objective The European League Against Rheumatism recommends implementing cardiovascular disease (CVD) risk assessments for patients with inflammatory joint diseases (IJDs) into clinical practice. Our goal was to design a structured programme for CVD risk assessments to be implemented into routine rheumatology outpatient clinic visits.
Methods The NOrwegian Collaboration on Atherosclerosis in patients with Rheumatic joint diseases (NOCAR) started in April 2014 as a quality assurance project including 11 Norwegian rheumatology clinics. CVD risk factors were recorded by adding lipids to routine laboratory tests, self-reporting of CVD risk factors and blood pressure measurements along with the clinical joint examination. The patients’ CVD risks, calculated by the European CVD risk equation SCORE, were evaluated by the rheumatologist. Patients with high or very high CVD risk were referred to their primary care physician for initiation of CVD preventive measures.
Results Data collection (autumn 2015) showed that five of the NOCAR centres had implemented CVD risk assessments. There were 8789 patients eligible for CVD risk evaluation (rheumatoid arthritis (RA), 4483; ankylosing spondylitis (AS), 1663; psoriatic arthritis (PsA), 1928; unspecified and other forms of spondyloarthropathies (SpA), 715) of whom 41.4 % received a CVD risk assessment (RA, 44.7%; AS, 43.4%; PsA, 36.3%; SpA, 30.6%). Considerable differences existed in the proportions of patients receiving CVD risk evaluations across the NOCAR centres.
Conclusion Patients with IJD represent a patient group with a high CVD burden that seldom undergoes CVD risk assessments. The NOCAR project lifted the offer of CVD risk evaluation to over 40% in this high-risk patient population.
- cardiovascular disease
- rheumatoid arthritis
- ankylosing spondylitis
- psoriatic arthritis
- health services research
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Statistics from Altmetric.com
- cardiovascular disease
- rheumatoid arthritis
- ankylosing spondylitis
- psoriatic arthritis
- health services research
Key messages
What is already known about this subject?
The implementation of cardiovascular disease (CVD) risk assessments for patients with inflammatory joint diseases (IJDs), recommended by the European League Against Rheumatism, has been underwhelming.
What does this study add?
The NOrwegian Collaboration on Atherosclerosis in patients with Rheumatic joint diseases (NOCAR) project is a nationwide, Norwegian quality assurance project aiming to implement CVD risk assessments for patients with IJD into routine rheumatology outpatient clinic visits with minimal extra resources and time cost.
How might this impact on clinical practice?
The NOCAR project lifted the offer of CVD risk evaluation to over 40% in this high-risk patient population, and we conclude that CVD risk assessments can be feasible as a part of daily rheumatology practice.
Introduction
Patients with inflammatory joint diseases (IJDs), including rheumatoid arthritis (RA), ankylosing spondylitis (AS) and psoriatic arthritis (PsA), have an increased risk of cardiovascular disease (CVD).1–4 This high CVD burden is related to the harmful effects of chronic inflammation on the vascular wall, and although the specific underlying mechanisms have not been entirely elucidated, it appears that persistently elevated inflammatory levels potentiate the detrimental effects of traditional CVD risk factors.5–7 Accordingly, it may be particularly important to monitor traditional CVD risk factors in patients with IJD. Nevertheless, patients with IJD patients appear to have a high prevalence of traditional CVD risk factors that are often suboptimally or not treated.8–14
The European League Against Rheumatism (EULAR) recommendations on CVD risk management in patients with IJD advocate regular CVD risk assessments for patients with IJD.1 15 However, the implementation of this recommendation into clinical practice has been underwhelming.8 9 16 17 Although a Danish study reported promising results from systematic CVD risk screening in designated nurse-led clinics, the feasibility of such initiatives are limited by high resource requirements.18 It has also been proposed that evaluation of CVD risk may be implemented into routine rheumatology clinical practice.1 19 Indeed, Gossec et al showed that CVD risk assessments in a routine rheumatology outpatient setting, including lipid values, smoking status and blood pressure (BP), anthropometric data and estimation of future CVD risk, can be undertaken in approximately 15 min.20 Moreover, we have previously shown that a structured, team-based and multidisciplinary approach can improve the rate of CVD risk factor recording almost three-fold.21
Our goal was to design a programme of annual CVD risk assessments that could be implemented into routine rheumatology outpatient clinic visits. Furthermore, we wanted to facilitate so that patients with IJD with increased CVD risk would receive guideline-recommended CVD preventive measures.22 In more general terms, the objective of this project was to increase the awareness of the high CVD burden in patients with IJD among rheumatology health personnel and patients. In the present project, we display our approach to CVD risk assessments in rheumatology outpatient clinics, as well as the capture rates of this method and the obstacles to successful implementation that we have encountered.
Methods
The nationwide quality assurance project, NOrwegian Collaboration on Atherosclerosis in patients with Rheumatic joint diseases (NOCAR), was initiated in April of 2014. The project includes 11 rheumatology clinics (figure 1) and comprises all four Norwegian health authorities. The project has been approved by the local Data Protection Officers as a quality assurance project (2014/11741).
Rheumatology centres in the NOCAR project. Oslo: Department of Rheumatology, Diakonhjemmet Hospital; Drammen: Department of Rheumatology, Drammen Hospital, Vestre Viken HF; Skien: Department of Rheumatology, Betanien Hospital; Kristiansand: Department of Rheumatology, Hospital of Southern Norway; Kristiansand: Revmatologene (specialist practice); Haugesund: Haugesund Rheumatism Hospital; Bergen: Department of Rheumatology, Haukeland University Hospital; Førde: Department of Rheumatology, Førde Central Hospital; Lillehammer: Lillehammer Hospital for Rheumatic Diseases; Trondheim: Department of Rheumatology, St. Olav’s University Hospital; Tromsø: Department of Rheumatology, University Hospital of North Norway. NOCAR, NOrwegian Collaboration of Atherosclerosis in patients with Rheumatic diseases.
The CVD risk assessment in NOCAR is facilitated by GoTreatIt Rheuma (GTI), an electronic system that collects and displays clinical information to patients and health personnel. The system facilitates the follow-up and monitoring of patients with IJD as part of ordinary clinical care (http://www.diagraphit.com/our-products/gotreatit-rheuma/). GTI was already in use in all participating centres prior to the start of the NOCAR project and also includes a designated CVD module.
CVD risk factor recording is accomplished in three steps in NOCAR (figure 2). First, the health secretaries add non-fasting lipid tests, including total cholesterol (TC), low-density lipoprotein cholesterol (LDL-c), high-density lipoprotein cholesterol (HDL-c) and triglycerides (TG), to the routine rheumatology laboratory tests. Second, patients self-report CVD risk factors (including diabetes mellitus, kidney disease, smoking and anthropometric measures for calculation of Body Mass Index), use of CVD preventive medication (eg, lipid-lowering agents and antihypertensive treatment) and presence of CVD comorbidities on electronic tablets connected to GTI in the waiting room prior to their consultation. Third, nurses perform BP measurements after a resting time of approximately 5–10 min and after the clinical joint examination. The nurses were instructed to make serial measurements in case of elevated BP levels and record the mean of the two last measurements provided that they differed <5 mm Hg. When the patients’ lipid and BP values are entered into GTI, the risk of experiencing a fatal CVD event in the coming 10 years, estimated by the systematic coronary risk evaluation (SCORE) algorithm, is automatically calculated.23 Subsequently, the rheumatologist can evaluate the SCORE estimate during the consultation. In accordance with the European Society of Cardiology guidelines for CVD prevention, although simplified, (1) when the estimated risk of future CVD was <5% (low to moderate risk), no further measures were taken, but a new CVD risk assessment would be performed the following year. (2) When the CVD risk estimate was >5% (high to very high risk), the patient was referred to the primary care physician (PCP) or a cardiologist by a standardised referral letter, explaining the indication for initiation of CVD preventive measures and/or treatment. Although the low-risk country SCORE algorithm is recommended for use in the Norwegian population,23 the rheumatologists were instructed to use the high-risk country SCORE algorithm to compensate for the increased CVD risk in patients with IJD.
Procedure for CVD risk factor recording and risk evaluation in patients with IJD in rheumatology outpatient clinics in the NOCAR project. BP, blood pressure; CVD, cardiovascular disease; IJD, inflammatory joint disease; NOCAR, NOrwegian Collaboration of Atherosclerosis in patients with Rheumatic diseases; SCORE, systematic coronary risk evaluation.
Rheumatology health personnel were also trained to deliver brief advice on smoking cessation and ‘heart-friendly’ diets. Moreover, information pamphlets on smoking cessation and healthy foods were made available in the clinics.
The results in this paper were retrieved from the NOCAR centres during the autumn of 2015 and include data from the first 1.5 years of the project. Eligibility criteria for patients participating in the NOCAR project were prespecified as any patient with a diagnosis of RA, AS, PsA or other forms of SpA, aged 30 to 80 years, who had a journal in GTI and visited a NOCAR centre during the project period. Patients who had available information on lipids, BP, smoking status, age and sex (the CVD risk factors included in the SCORE equation) were defined as having undergone a CVD risk assessment.
Statistics
The capture rate in the NOCAR project was calculated as the number of patients who had undergone CVD risk assessments divided by the total number of eligible patients. Separate analyses were performed for each NOCAR centre, for the individual diagnoses, as well as for the whole population.
The CVD evaluation in NOCAR was constructed so that lipid tests were added to routine rheumatology laboratory tests. However, a proportion of the patients who were counted as eligible for the NOCAR project did not have routine rheumatology laboratory tests taken during the project period and, thus, lipid measurements were not feasible. Moreover, some patients who were tallied as eligible were in fact in an investigatory rheumatology process to verify or reject a rheumatology diagnosis and therefore did not actually qualify for the NOCAR project. Taking this into account, and to find a more correct number of eligible patients, we performed additional analyses that included only patients who in addition to the formerly mentioned eligibility criteria also had (1) measured routine rheumatology laboratory tests and/or (2) documented current or previous use of antirheumatic medications.
The characteristics of the patients who were included in NOCAR (ie, those who had undergone a CVD risk assessment, including lipid tests, self-reporting and BP measurements) and those who were not included in the project (ie, patients for whom lipid tests, self-reporting and/or BP measurements were lacking) were expressed as number (%) for dichotomised variables, as well as mean±SD and median with IQR for normally and non-normally distributed variables, respectively. The patient characteristics were also compared across the two groups using analysis of variance, and χ2 tests as appropriate. Non-normally distributed variables were logarithmically transformed before comparison.
Results
During the time period from April 2014 to the autumn of 2015, 7 of the 11 NOCAR centres had implemented CVD risk assessments into clinical practice. The reasons that the four (Trondheim, Haugesund, Revmatologene (specialist practice), Førde) remaining centres had not started were mainly related to shortage of clinical personnel/resources at the time of project implementation. In addition, two (Bergen, Skien) of the seven centres that commenced CVD risk assessments only performed test runs of the project on certain days. Accordingly, we only report on data retrieved from the remaining five centres, Diakonhjemmet Hospital (Oslo), Hospital of Southern Norway (Kristiansand), Lillehammer Hospital for Rheumatic Diseases (Lillehammer), Drammen Hospital (Drammen) and University Hospital of North Norway (Tromsø), which had a total of 8789 patients who were eligible for the NOCAR project (Oslo, n=1972; Lillehammer, n=1872; Kristiansand, n=2121; Drammen, n=1240; Tromsø, n=1584). In detail, there were 4483 patients with RA, 1663 patients with AS, 1928 patients with PsA and 715 patients with other forms of SpA, who were eligible for participation in the NOCAR project. Data on the CVD risk profiles, risk estimations and CVD risk factor treatment in the NOCAR project have already been published and are available elsewhere.24–26
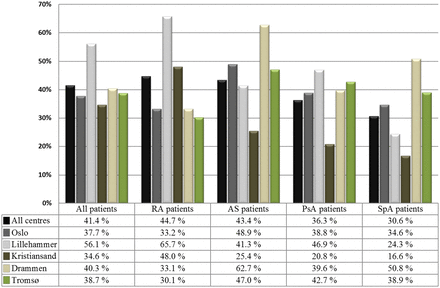
Overall, 41.4% of the eligible patients received a CVD risk assessment during the first 1.5 years of the project (figure 3). In the additional analyses, excluding patients who did not have routine rheumatology laboratory tests during the project period, and those who had never used antirheumatic medications, the CVD risk assessment capture rate increased to 48.5% (online supplementary material). There were considerable discrepancies in the capture rates across the IJD diagnoses, ranging from 44.7% of patients with RA to 30.6% among those with other forms of SpA (figure 2). We also found differences in the capture rates across the NOCAR centres, ranging from 56.1% in Lillehammer to 34.6% in Kristiansand.
Cardiovascular disease risk assessment capture rates in the NOCAR project, evaluated across diagnosis groups and participating centre. AS, ankylosing spondylitis; NOCAR, NOrwegian Collaboration of Atherosclerosis in patients with Rheumatic diseases; PsA, psoriatic arthritis; RA, rheumatoid arthritis; SpA, other spondyloarthropathies.
Comparisons of demographic and socioeconomic data (table 1) show that patients who underwent a CVD risk assessment were generally comparable witho those who did not, the most notable exception being that the patients who were included in NOCAR were more often working and less often pensioners.
Patient characteristics: —demographic and socioeconomic variables compared across patients who received cardiovascular disease risk assessments in the NOCAR project and those who did not
Variables expressed as number (%) for dichotomous variables, mean±SD for normally distributed variables and median (IQR) for non-normally distributed variables.
Furthermore, there were significant discrepancies with regards to rheumatology disease–related variables (table 2). Overall, compared with those who had not undergone a CVD risk assessment, patients who had received CVD risk evaluations had longer disease duration and lower disease activity (acute phase reaction indicators, patient’s and investigator’s global assessment, swollen/tender joints, disease activity score in 28 joints and Clinical Disease Activity Index scores). On the other hand, the patients who underwent CVD risk assessments were more often current or former users of biologic disease-modifying antirheumatic drugs (bDMARDs) and synthetic DMARDS, whereas they were less often current users of prednisolone. Use of non-steroidal anti-inflammatory drugs is not presented due to poor reporting rates.
Patient characteristics: rheumatology disease variables compared across patients who received cardiovascular disease risk assessments in the NOCAR project and those who did not
Discussion
Through the nationwide NOCAR project, over 3600 patients with IJD have received CVD risk assessments that they would not otherwise have been offered in their rheumatology outpatient clinic. Thus, we have shown that implementation of CVD risk assessment into daily rheumatology practice can be feasible. Still, a capture of 40%–50% shows that this change process is complicated and will take time to optimise. In the following sections, we discuss hindrances and success factors that we encountered in the NOCAR project.
However, we exposed large differences in the capture rates during the first 1.5 years of the project. The observed capture discrepancies across the different IJD diagnoses may be attributable to better knowledge among health personnel about the increased CVD risk in patients with RA, compared with the less well-known associations between CVD and PsA, AS and other forms of SpA. There were also significant variances in the capture rates across the NOCAR centres. These discrepancies were observed as a function of the timing of the implementation of the NOCAR project in the various clinics. Lack of available resources due to shortage of health personnel or competing projects/studies were the major explanations for why several centres had to postpone implementation of CVD risk assessments. It should be noted that there were only minor differences in demographic and socioeconomic data between the patients who received a CVD risk assessment and those who did not. Accordingly, it is unlikely that patients were selected for the project on the basis of factors that are not directly related to their disease. Conversely, patients with high disease activity were less likely to undergo CVD risk assessments, implying that rheumatologists address comorbidities (including CVD) when low disease activity or remission has been obtained. This may seem counterproductive, considering that inflammation and CVD risk is strongly correlated.6 On the contrary, and as stated in the 2009 EULAR recommendations, adequate control of disease activity is pivotal to lower the CVD risk.1 Furthermore, the estimated risk of future CVD may be artificially low when the disease activity is high due to the inverse relationship between inflammation and lipid levels.27
Many potential barriers to implementation of CVD risk assessments in rheumatology have been proposed,13 19 20 28 29 several of which were encountered during the implementation of the NOCAR project. First, CVD risk assessments traditionally belong to cardiologists/PCPs and represent novel elements in already busy rheumatology outpatient clinics. Furthermore, rheumatology health personnel may have the impression that CVD risk evaluation is time consuming. However, we maintain that CVD risk assessments may be performed expediently if they are properly implemented, and it has been argued that the time spent on CVD risk assessments is likely to decrease with growing experience.20
Second, there are uncertainties regarding whether the CVD aspect of IJD is a concern that should be attended to by rheumatologists, PCPs or cardiologists.13 It is an overarching principle in the recently published EULAR recommendations for CVD risk management that the rheumatologist is responsible for ensuring that CVD risk evaluations are undertaken.15 In most countries, CVD risk assessments and initiation of CVD preventive measures are among the main responsibilities of the PCPs. In fact, a recent study by Weijers et al , in which lipid measurements were used as a proxy for CVD risk assessments, indicates that if one establishes collaborations between PCPs and rheumatologists, the responsibility of CVD risk assessments may be very well suited with the PCP.30 However, the increasing number of guidelines/recommendations makes it difficult to be aware of every applicable instruction.31–33 Indeed, lack of awareness and familiarity with clinical guidelines are major barriers to successful implementation of evidence-based medicine.31 Along the same lines, Bell et al found that less than one in three PCPs recognise RA as an independent risk factor for CVD, and that only 15% of PCPs assessed the CVD risk of their patients with RA.34 Furthermore, many patients with IJD may not see their PCP regularly, and this has been shown to predict a lack of annual lipid measurements.8 When taking these elements into account, we maintain that CVD risk screening procedures are best suited with the rheumatologist and, as we have shown, in the rheumatology outpatient clinic.
Third, the rheumatologist may have the perception that when a patient with increased CVD risk is identified, it will be their responsibility to initiate CVD preventive measures that they are not used to supervise. We hold the opinion that this challenge can be solved by collaborating with a PCP or a cardiologist who can take charge of the CVD preventive interventions after the CVD risk assessment has been undertaken.
Besides the three aforementioned general challenges to successful implementation, we encountered several practical barriers to CVD risk assessments. For instance, there were challenges related to defining a date for the annual CVD risk assessment, and especially for patients who had several visits per year and at irregular time intervals. Interestingly, Akenroye et al found that electronic medical record–based support tools that reminded rheumatologists about the date of the last CVD risk assessment did not lead to improved CVD risk factor capture rates.35 It is our experience that the capture rates may be improved if CVD risk assessments are linked to other annual rheumatology reviews or to annual follow-ups in other studies/projects. For instance, the NOCAR project was coupled to the Norwegian Antirheumatic Drug Register (NOR-DMARD) in two of the centres, in which biannual visits are well incorporated into the clinical routine.36 The coupling of the NOCAR project to the NOR-DMARD register explains the high rates of bDMARD use among the patients who underwent CVD risk assessments in the NOCAR project. In the recently published EULAR recommendations for CVD risk management in patients with IJD, the time intervals between CVD risk screenings were increased from 1 to 5 years.15 This modification is likely to entail further challenges with regards to defining a date for the CVD risk assessment. Moreover, when designing strategies to implement 5-yearly CVD risk assessments according to the latest EULAR recommendations, one may have to acknowledge that 100% annual capture rates are not feasible in rheumatology outpatient clinic settings and that annual capture rates are more likely to be comparable with those that we have observed in the NOCAR project (ie, approximately 30%–40%).15
Another hindrance for successful implementation of CVD risk evaluation that we encountered was related to the patient’s travelling distances to the rheumatology outpatient clinics. Several of the NOCAR centres hold regional rheumatology responsibilities, and accordingly, many patients might not have their lipids measured in the hospital laboratory prior to the consultation. One possible solution to this problem would be to ask the patient to measure their lipids in advance of their appointment and bring the results to the rheumatology consultation.
The 2009 EULAR recommendations for CVD management in patients with IJD stated that a 1.5 multiplier should be applied to the CVD risk estimates of patients with RA with certain disease characteristics to make up for their increased CVD burden.1 However, we judged that introducing one more factor to the risk calculation could obstruct successful implementation of CVD risk assessments. To compensate for the increased risk of CVD, we instead instructed the rheumatologists to use the SCORE algorithm for high-risk countries, which yields approximately 1.6–1.7 times higher CVD risk estimates compared with the low-risk country SCORE algorithm. A further limitation related to the SCORE algorithm was that the calculator was applied to all patients, regardless of age and comorbidities, such as diabetes, hypertension and kidney failure. With regards to age, the SCORE equation has an upper age limit of 65 years. Thus, for patients aged >65 years, the SCORE risk estimate of a patient of 65 years with the same CVD risk factor levels was used. This approach, although suboptimal and unspecific, was chosen to increase the feasibility of the project, as it was expected that too many details would reduce the rheumatologists’ inclination to perform CVD risk assessments. The same rationale was the basis for omitting measurements of fasting blood glucose or glycated haemoglobin. However, the addition of diabetes screening has been discussed as a potential addition to the NOCAR project in the future.
The use of non-fasting lipids in lieu of fasting lipids may appear suboptimal. However, requiring fasting laboratory samples would reduce the feasibility of the NOCAR project, and we refer to large epidemiological studies showing how TC and HDL-c levels (the lipid fractions included in SCORE) are comparable in fasting and non-fasting individuals.37 38 One should, however, be aware that TG levels depend on the prandial state and that the same holds true for LDL-c if it is calculated by Friedewald’s formula.
It may appear that the use of GTI, which facilitated the calculation of CVD risk estimates by SCORE, limits the generalisability of the results. However, the existence of CVD risk charts and online CVD risk calculators (eg, www.heartscore.org) facilitates effective implementation of CVD risk screening without electronic patient journals such as GTI. Otherwise, the project was carried out in the normal rheumatology clinical setting of several different hospitals that had no specific orientation towards cardiology, which implies a good level of generalizability.
Since the data presented in this paper are based on data from the first 1.5 years of the NOCAR project, we do not know if the project has led to significant changes in CVD risk. In other words, we do not know if the high-risk patients who were identified were actually started on CVD preventive medication. However, we plan to make serial data extractions to evaluate if the intervention has been successful, both in terms of reducing CVD risk and in terms of reducing the rate of CVD events in this population.
The results in this paper were retrieved from the NOCAR centres during the autumn of 2015 and include data from the first 1.5 years of the project. Eligibility criteria for patients participating in the NOCAR project were prespecified as any patient with a diagnosis of RA, AS, PsA or other forms of SpA, aged 30 to 80 years, who had a journal in GTI and visited a NOCAR centre during the project period. Patients who had available information on lipids, BP, smoking status, age and sex (the CVD risk factors included in the SCORE equation) were defined as having undergone a CVD risk assessment.
In conclusion, the NOCAR project lifted the offer of CVD risk evaluation to over 40% in a high-risk patient population for whom such evaluations did not previously exist. Through a Norwegian nationwide project, we have shown that CVD risk assessments can be feasible as a part of a daily rheumatology practice, but that this requires a change process that is likely to require some time before they are functioning optimally. The NOCAR project will continue, with the goal of optimising the percentage of patients having their CVD risk evaluated even further. We hope that similar strategies can be implemented in other countries in order to improve CVD prevention in patients with IJD.
References
Footnotes
Contributors We confirm that all authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship and have approved this manuscript for submission.
Funding The work was supported by the South-Eastern Norway Regional Health Authority (grant no. 2013064).
Competing interests GH is founder and shareholder of the company DiaGraphIT, manufacturing GoTreatIT Rheuma. The other authors have received no financial support that could create a potential conflict of interests, or the appearance of such, with regard to the work.
Patient consent Not required.
Ethics approval The project has been approved by the local Data Protection Officers as a quality assurance project (2014/11741).
Provenance and peer review Not commissioned; externally peer reviewed.
Data sharing statement Some of the data from this project may be shared on request to the corresponding author.