Article Text
Abstract
Objective The role of interferons (IFN) in the pathophysiology of primary inflammatory and dysimmune myopathies (IDM) is increasingly investigated, notably because specific neutralisation approaches may constitute promising therapeutic tracks. In present work we analysed the muscular expression of specific IFNα/β and IFNγ-stimulated genes in patients with various types of IDM.
Methods 39 patients with IDM with inclusion body myositis (IBM, n=9), dermatomyositis (DM, n=10), necrotising autoimmune myopathies (NAM, n=10) and antisynthetase myositis (ASM, n=10), and 10 controls were included. Quantification of expression levels of IFNγ, ISG15, an IFNα/β-inducible gene and of six IFNγ-inducible genes (GBP2, HLA-DOB, HLA-DPB, CIITA, HLA-DRB and HLA-DMB) was performed on muscle biopsy samples.
Results DM usually associated with strong type I IFNα/β signature, IBM and ASM with prominent type II IFNγ signature and NAM with neither type I nor type II IFN signature. Immunofluorescence study in ASM and IBM showed myofibre expression of major histocompatibility class 2 (MHC-2) and CIITA, confirming the induction of the IFNγ pathway. Furthermore, MHC-2-positive myofibres were observed in close proximity to CD8+ T cells which produce high levels of IFNγ.
Conclusion Distinct IFN signatures allow a more distinct segregation of IDMs and myofibre MHC-2 expression is a reliable biomarker of type II IFN signature.
- interferon
- ISG15
- major histocompatibility class 2 (MHC-2)
- dermatomyositis
- inclusion body myositis
- necrotising autoimmune myopathies
- antisynthetase
- inflammatory myopathy
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
- interferon
- ISG15
- major histocompatibility class 2 (MHC-2)
- dermatomyositis
- inclusion body myositis
- necrotising autoimmune myopathies
- antisynthetase
- inflammatory myopathy
Key messages
What is already known about this subject?
Among inflammatory/dysimmune myopathies (IDMs), dermatomyositis (DM) is the only associated with type I-interferon signature.
Most IDMs are associated with myofiber expression of major histocompatibility complex (MHC)-class I. MHC-I is induced by interferons suggesting a possible role for type II-interferon in IDMs other than DM.
What does this study add?
In this study, we showed that myofiber MHC-II expression is observed in inclusion body myositis (IBM) and antisynthetase myositis (ASM), but not in DM and necrotizing autoimmune myopathy (NAM).
In accordance with this finding, we showed that IBM and ASM are specifically associated with Type-II IFNγ signature, DM only with Type-I IFN signature, and NAM with neither Type-I nor Type-II IFN signature.
How might this impact on clinical practice?
Distinct IFN signatures allow a more distinct segregation of IDMs and therefore a more accurate diagnosis.
Deciphering IFN signatures in IDMs will also lead to develop new therapeutic approaches targeting IFNs pathways.
Introduction
Both muscle biopsy and circulating myositis-specific autoantibodies (MSA) are used in the diagnostic workup for inflammatory and dysimmune myopathies (IDM). Muscle biopsy made possible to visualise and classify the pathological processes leading to myofibre injuries, such as CD8 T cell-mediated cytotoxicity in inclusion body myositis (IBM), endomysial microangiopathy with ischaemic myofibre injuries in dermatomyositis (DM) and complement-mediated myofibre necrosis in necrotising autoimmune myopathies (NAM).1 MSAs had a major implication in delineation of distinctive patterns of disease or phenotype and in predicting prognosis of IDMs.2 In addition, the role of interferons (IFN) in the pathophysiology of IDMs is increasingly scrutinised in a therapeutic perspective,3 notably because it may lead to specific IFN neutralisation approaches.4 For example, DM displays a strong type I IFNα/β signature.5 6 In contrast to DM, the role of IFNs has not been well established in other types of IDM.7
In a previous study on anti-synthetase syndrome-associated myositis (ASM), a condition in which muscular involvement is associated with peculiar extramuscular features and specific circulating autoantibodies, we observed that, in contrast to DM, there was conspicuous myofibre expression of major histocompatibility class 2 (MHC-2) antigens.7 Since MHC-2 expression by myogenic cells is induced by type II IFNγ and blocked by type I IFNα/β,8 marked MHC-2 expression in ASM suggested imbalance between IFN type I and type II pathways in favour of IFN type II in this specific IDM subtype. In the present study, type I and type II IFN signatures were determined in muscle biopsy samples from patients with the main IDM subtypes, including DM, IBM, NAM and ASM. Expression analysis of specific IFNα/β and IFNγ-stimulated genes characterised DM as specifically associated with type I IFNα/β signature, IBM and ASM with type II IFNγ signature and NAM with neither type I nor type II IFN signature.
Materials and methods
Patients and muscle samples
Muscle biopsies from patients with NAM (n=10), DM (n=10), ASM (n=10), IBM (n=9) and histologically normal muscle (n=10, controls) were obtained from the Department of Pathology, Henri Mondor University Hospital (Créteil, France). A polymyositis (PM) group was not included because of the extreme rarity of idiopathic PM when strict diagnostic criteria are used. This study was conducted in accordance with French rules (Henri Mondor Biological Resource Platform: registration number DC-2009-930, French Ministry of Research). Autoantibodies screening was carried out using commercially available linear dots. All patients had muscle biopsy for diagnostic purposes, with immunoperoxidase staining of MHC-1 and MHC-2 antigens, complement membrane attack complex (C5b-9), CD56/NCAM (myofibre regeneration), CD68 (macrophages), and CD3, CD4, CD8 and CD20 (leucocyte subsets), and immunofluorescence for dual labelling (MHC-2/class II MHC transactivator (CIITA); MHC-2/CD8) (references in online supplementary information). European Neuromuscular Centre (ENMC) criteria were used to diagnose DM, NAM and IBM, and Troyanov classification was used to diagnose overlap myositis,1 the ASM subset of which being defined by detection of circulating antisynthetase autoantibodies. Myofibre MHC-2 expression was defined by sarcolemmal staining with or without sarcoplasmic staining.7
Supplemental material
Real-time quantitative PCR
RNAs were extracted from biopsy samples using Trizol reagent and quantified by NanoDrop. cDNAs were synthesised using the Maxima First strand cDNA synthesis kit (Thermo Scientific). We analysed the expression levels of IFNγ, ISG15, an IFNα/β-inducible gene and of six IFNγ-inducible genes: GBP2, HLA-DOB, HLA-DPB, CIITA, HLA-DRB and HLA-DMB. We used quantitative RT-PCR on ABI PRISM 7900HT using TaqMan assays (Applied Biosystems) or the SYBR Green reagent. Assays and primer sequences are indicated in online supplementary information). The median fold change in expression of six IFNγ-stimulated genes for each patient biopsy sample was used to define an IFNγ score.9
Statistical analysis
Statistical analyses were performed using the non-parametric Mann-Whitney test (p<0.05 was considered significant) and GraphPad Prism V.6.0 (GraphPad Software, La Jolla, CA).
Results
Patient characteristics
Patients with ASM included four women and six men (mean age: 55 years; range: 25–83), with anti-Jo1 autoantibodies in 9/10 and anti-PL7 in 1/10. Patients with DM included eight women and two men (mean age: 58 years; range: 34–85), and patients with IBM included four women and five men (mean age: 69 years; range 61–89). Patients with NAM included eight women and two men (mean age: 54 years; range 24–90), with anti-signal recognition particle autoantibodies in 3/10, anti-HMGCR in 3/10 and anti-Ku in 1/10.
IFNα/β-inducible gene expression in muscle tissues
We first tested the involvement of type I IFNα/β in the various IDM subsets by quantifying the level of ISG15 expression, an IFNα/β-inducible gene in muscle. ISG15 expression was markedly and specifically increased in DM: DM versus NAM: p<0.0001; DM versus ASM: p<0.0001; DM versus IBM: p<0.0001 (figure 1H).
Relative expression of IFNγ (A), CIITA (B), HLA-DRB (C), HLA-DMB (D), HLA-DOB (E), HLA-DPB (F), GBP2 (G) and ISG15 (H) in NAM, DM, ASM and IBM. *P<0.05; **P<0.01; ***P<0.001. ASM, anti-synthetase syndrome-associated myositis; DM, dermatomyositis; IBM, inclusion body myositis; IFN, interferon; NAM, necrotising autoimmune myopathies; RQ, relative quantification.
Expression IFNγ and its inducible genes in muscle tissue
IFNγ expression in muscle varied according to each IDM subtype. The highest levels of IFNγ were observed in patients with IBM followed by patients with ASM, whereas IFNγ expression was detectable at low level in DM, and absent (comparable to controls) in NAM (figure 1A).
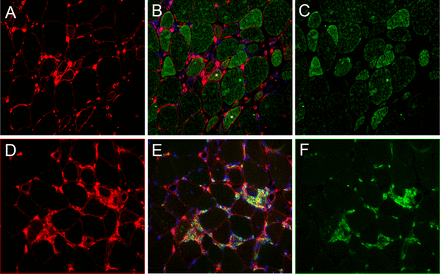
Consistently expression of the IFNγ-inducible CIITA gene tended to be higher in ASM and IBM compared with NAM and DM (figure 1B). Immunohistochemical expression of the IFNγ-inducible MHC-2 differed between ASM and IBM, mainly perifascicular in ASM and multifocal in IBM (figure 2). Expression levels of HLA-DOB and HLA-DPB genes were significantly higher in IBM compared with NAM (p=0.0039 and p=0.0002, respectively) (figure 1E,F), and in ASM compared with NAM (p=0.0027 and p=0.0051, respectively) (figure 1E,F). Expression levels of HLA-DRB genes were significantly higher in IBM compared with NAM (p=0.0433) but not in ASM (figure 1C). Increased HLA-DPB gene expression could segregate both IBM and ASM from DM (p=0.002 and p=0.0028, respectively) (figure 1F). HLA-DQ gene expression level fell beneath the detection threshold. In contrast, increased GBP2 gene expression could discriminate both IBM and ASM from NAM (p=0.0524 and p=0.0021, respectively), and ASM from DM (p=0.0062) (figure 1G).
Muscle biopsy from a patient with inclusion body myositis (IBM). (A–C) Immunofluorescence staining of major histocompatibility class 2 (MHC-2) and CIITA: MHC-2, red (A); CIITA, green (C); merged with DAPI for nuclei staining in blue (B). The pattern of MHC-2-positive myofibre distribution appears multifocal. Double staining shows CIITA-positive/MHC-2-negative myofibres corresponding to an early step of interferon-γ (IFNγ) signalling, double-positive CIITA/MHC-2 fibres (stars) indicating early MHC-2 transactivation and CIITA-negative/MHC-2-positive fibres indicating later stages, concomitant with CIITA degradation. (D–F) Immunofluorescence staining of MHC-2 and CD8: MHC-2, red (D); CD8, green (F); merged with DAPI for nuclei staining in blue (E). CD8+ T cells are found proximal to MHC-2-positive myofibres, MHC-2 expression by CD8 T cells (double-stained cells), (E) indicating activated state. Frozen muscle sections (7 µm). Magnification ×10.
In line with these results, the IFNγ score defined by the median fold change in expression of six IFNγ-induced genes clearly discriminated ASM and IBM from NAM (p=0.0022 and p=0.0022, respectively) and ASM and IBM from DM (p=0.0411 and p=0.0043).(figure 3) Taken together, these data were consistent with an IFNγ signature in ASM and IBM.
IFN score calculated from the median fold change in expression of six IFNγ-induced genes (CIITA, HLA-DRB, HLA-DMB, HLA-DOB, HLA-DPB, GBP2).13 *P<0.05; **P<0.01; ***P<0.001. IFN, interferon.
IFNγ pathway activation in myofibres
The respective localisation of MHC-2 and CIITA expressions was assessed by immunofluorescence in IDM subsets with elevated IFNγ score, that is, ASM and IBM. In ASM, MHC-2 and CIITA expressions were primarily observed in perifascicular myofibres (figure 2A). Most myofibres coexpressed MHC-2 and CIITA, with occasional fibre expression MHC-2 or CIITA alone (figure 2A). In contrast, in IBM, MHC-2-positive myofibres were distributed in multifocal clusters that coexpressed CIITA (figure 2A). CD8-positive cells known to produce IFNγ were usually observed in the vicinity of MHC-2-positive myofibres (figure 2B). In DM, only few MHC-2-positive myofibres were observed. In NAM, no myofibre MHC-2 expression was observed, and no or very few CD8-positive cells were detected in the endomysium.
Discussion
Many reports have reported type I IFNα/β signature in muscle and blood of patients with DM.10 11 Consistently, the type I IFN-inducible ISG15 gene expression was upregulated in muscle of our patients with DM as previously documented by microarray analyses in whole muscle5 12 or microdissected MHC-1-positive myofibres.13 In DM, perifascicular atrophic fibres also strongly express MxA protein, an important type I IFN-related factor.5 The inducer of type I IFN release is unknown in DM, but IFNα and β are known to be produced by plasmacytoid dendritic cells that are increased in DM muscle.5 Type I IFNs have been recognised to play key roles in the initiation and maintenance of the DM process, through exacerbation of inflammatory responses,14 upregulation of MHC-1 in myofibres, effects on endothelial cells5 and induction of mitochondrial dysfunction.15
To assess type II IFN, we used a panel of genes including IFNγ, CIITA, GBP2 and four MHC-2 genes. This combination was chosen to focus on genes electively induced by IFNγ despite highly weight with MHC-2-related transcripts. Other IFNγ-induced genes could be used such as GBP5, ICAM1, CAMK2D, IRF1, SOCS3, CD44 or CCL2.16 In accordance with the existence of CD8-positive large granular lymphocytes in sIBM,10 we showed that type II IFN signature was electively increased in sIBM. IFNγ is a potent inducer of MHC-2 expression,7 an effect mediated by the transactivator CIITA.11 Increased muscle expression of IFNγ in ASM and IBM was associated with both CIITA gene upregulation and myofibre expression of MHC-2 antigens. In DM, the type II signature was low but not null, in keeping with the presence of few numbers of MHC-2-positive myofibres.7 Previous studies investigating myofibre MHC-2 expression in DM17 18 showed a median percentage of MHC-2-positive fibres ranging from 2.2% to 8.6%,17 a proportion similar to that we reported (7.5%)7 and indicating that type I and type II signatures may occasionally overlap in DM. In contrast, NAM expressed virtually no type I or type II IFN signature and no MHC-2 in myofibres, a feature in agreement with current view of NAM pathogenesis highlighting a role for autoantibodies and complement activation in myofibre injuries.193
The main sources of IFNγ are natural killer (NK) cells, CD4-Th1 and CD8-cytotoxic T lymphocytes.20 In addition to type II IFN signature, we observed CD8+ T cells in close vicinity of MHC-2 expressing myofibres in both ASM and IBM (figure 3). NK cells are rare in muscular inflammatory infiltrates in ASM21 and virtually absent in IBM.22 This finding points out CD8+ T cells as a likely source of local IFNγ production in ASM and IBM. In sIBM, IFNγ signalling cascade was shown upregulated in attacked myofibres but not in non-attacked supporting this view.23 Expression of CIITA protein was restricted to myofibres in ASM and IBM (figure 2A). In addition to inducing MHC-2, and possibly MHC-1 myofibre expression, IFNγ may also inhibit myogenesis through CIITA by recruiting myogenin together with silencing epigenetic factors to target gene promoters.24 So, IFNγ activity may both sustain inflammation and impair injured myofibre repair, and therefore could aggravate the MHC-1/TCR-mediated myofibre destruction by CD8 T cells.
We hope that the distinctive IFN signatures in the different IDM subtypes reported herein could help in further development of key molecular pathway-based therapeutic approaches, targeting IFN type I in DM,3 25 IFN type II in ASM and IBM, and complement-mediated cell death in NAM.26
Acknowledgments
CH and BP benefited from a fellowship from Région Ile-de-France and RHU CARMMA, respectively.
References
Footnotes
Contributors MR performed RTqPCR, analysed results and wrote the manuscript. CH analysed results, designed the figures, performed immunostaining and participated in the writing of paper. YBA performed immunostaining. JA carried out clinical data and histopathological analyses. BP managed biobanking and performed immunohistochemistry. RKG contributed to the redaction of paper. PL analysed data and participated in the redaction of paper. FJA was in charge of conception, supervision, data analyses and redaction of the paper.
Funding This work was funded by grants from Association Française contre les Myopathies (AFM), Pole Translamuscle (project number 19507), MyoDR (project number 19100).
Competing interests None declared.
Patient consent for publication Obtained.
Ethics approval French Ministry of Research (authorisation number DC-2009-930).
Provenance and peer review Not commissioned; externally peer reviewed.
Data sharing statement There is no data repository for sharing.