Article Text
Abstract
Objectives Although current treatment guidelines for rheumatoid arthritis (RA) suggest tapering disease-modifying anti-rheumatic drugs (DMARDs), it is unclear whether DMARD-free remission (DFR) is an achievable and sustainable outcome. Therefore, we systematically reviewed the literature to determine the prevalence and sustainability of DFR and evaluated potential predictors for DFR.
Methods A systematic literature search was performed in March 2019 in multiple databases. All clinical trials and observational studies reporting on discontinuation of DMARDs in RA patients in remission were included. Our quality assessment included a general assessment and assessment of the description of DFR. Prevalence of DFR and its sustainability and flares during tapering and after DMARD stop were summarised. Also, potential predictors for achieving DFR were reviewed.
Results From 631 articles, 51 were included, comprising 14 clinical trials and 5 observational studies. DFR definition differed, especially for the duration of DMARD-free state. Considering only high- and moderate-quality studies, DFR was achieved in 5.0%–24.3% and sustained DFR (duration>12 months) in 11.6%–19.4% (both relative to the number of patients eligible for tapering). Flares occurred frequently during DMARD tapering (41.8%–75.0%) and in the first year after achieving DFR (10.4%–11.8%), while late flares, >1 year after DMARD-stop, were infrequent (0.3%–3.5%). Many patient characteristics lacked association with DFR. Absence of autoantibodies and shared epitope alleles increased the chance of achieving DFR.
Conclusions DFR is achievable in RA and is sustainable in ~10%–20% of patients. DFR can become an important outcome measure for clinical trials and requires consistency in the definition. Considering the high rate of flares in the first year after DMARD stop, a DMARD-free follow-up of >12 months is advisable to evaluate sustainability.
- DMARD-free remission
- disease flare
- rheumatoid arthritis
- tapering
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
INTRODUCTION
In rheumatoid arthritis (RA), early treatment, with disease-modifying antirheumatic drugs (DMARDs), aiming at sustained remission, is nowadays the key element of each management approach.1 2 As a result, RA has become a controllable disease in which sustained clinical remission is achievable for an increasing number of patients, and tapering and discontinuation of DMARDs have become of emerging interest.3 Current international guidelines recommend tapering of DMARDs in RA patients with sustained remission.1 2 Nevertheless, these guidelines are less clear whether DMARDs can be stopped, and the systematic literature review supportive of the most recent EULAR guidelines was not focused on DMARD cessation.4
Key messages
What is already known about this subject?
Although current treatment guidelines for rheumatoid arthritis suggest tapering DMARDs when patients are in sustained remission, it is unclear whether DMARD-free remission is an achievable and sustainable outcome.
What does this study add?
DMARD-free remission is achievable in rheumatoid arthritis and is sustainable in ~10%–20% of patients.
How might this impact on clinical practice or future developments?
DMARD-free remission can become an important outcome measure for clinical trials, though this requires consistency in the definition.
We propose to incorporate a DMARD-free follow-up period for at least 1 year, to ensure that DMARD-free remission is sustainable.
Despite the recommendations in the guidelines, tapering of DMARDs has not been adopted structurally in many clinical practices, presumably because the risk of a disease flare5 and because the ability to achieve and sustain DMARD-free remission (DFR) is often considered unlikely.6 On the other hand, there is increasing interest in achieving DFR, because this is currently the best proxy for cure.7 8 Clinical trials occasionally report on DFR, but usually not as the primary outcome. Absence of knowledge of DFR prevalence, its sustainability and the characteristics of patients achieving DFR currently hamper the use of DFR as primary outcome.9
We aimed to expand the comprehension of the ability to achieve and sustain DFR in RA. Therefore, we conducted a systematic literature search. In addition to the DFR prevalence and sustainability, potential predictors for achieving DFR were explored.
METHODS
Search strategy and selection criteria
This systematic literature review was conducted in accordance with the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) guidelines and the Cochrane review handbook.10 11 The protocol was registered in the International Prospective Register of Systematic Reviews (CRD42019132558).12
The search strategy was developed and performed in collaboration with an experienced librarian (JS). Key terms used for the search were ‘Rheumatoid arthritis’, ‘Antirheumatic drugs’, ‘Discontinuation’ and ‘Remission’. These search items were translated into multiple matching synonyms in order to broaden our results. All search elements were combined with the Boolean operators AND/OR. PubMed, Embase, Web of Science, COCHRANE Library, Emcare and Academic Search Premier were systematically searched (supplementary table S1).
Supplemental material
All observational cohorts and clinical trials reporting on discontinuation of DMARDs in RA patients, in remission, were included. Study selection was independently carried out by two reviewers (MV and EvM). Cases of disagreement were discussed until consensus was reached. First, all obtained titles were screened, and subsequently abstracts were reviewed after which full-text articles were screened for the predefined inclusion and exclusion criteria (supplementary table S2). If multiple articles were based on the same study, the article which described the prevalence and sustainability of DFR most clearly was selected. Subsequently, the article describing the longest follow-up was used for data extraction.
Data extraction
A standardised data collection form was used to extract the following information: study design, patient characteristics, interventions, glucocorticoids (GCs) usage, organisation of follow-up, outcome measures and loss to follow-up (supplementary table S3). Furthermore, data regarding eligibility criteria for tapering, tapering methods, numbers of patients tapering, description and timing of achieving DFR, sustainment of DFR over time and the occurrence of flares were extensively explored. Also, information regarding predictors of DFR was collected. Data extraction was done independently by two reviewers (MV and EvM), and disagreements were discussed until consensus was reached.
If the methods were incomplete or unclear, the methods of the original study could be used if a reference was available. Clinical trials and observational studies were handled separately, because of fundamental differences in the study design, which could influence achievement and sustainment of DFR, that is, protocolised versus non-protocolised tapering, frequency of monitoring and duration of follow-up.
Quality assessment
Our study quality assessment consisted of two parts, namely a general assessment and an assessment of the description of DFR. For the general quality assessment, we used 13 predefined quality criteria, which were based on Cochrane guidelines (supplementary table S4).11 The general study quality was considered ‘good’ if >75% (≥10 items) of these criteria were scored positive. For the DFR quality assessment, we used the following criteria: (1)‘DFR definition’, referring to whether a definition (eg, remission criterion) of DFR was included, and (2)‘DFR duration’, referring to whether information on the time between DMARD stop and being appointed as DFR (i.e. the duration of DMARD-free status) was reported. Specific emphasis was put on the duration of DMARD-free state since this attains insight into the sustainability of DFR. When both DFR quality criteria were scored positive, DFR quality was regarded as ‘good’.
Studies were regarded as ‘high quality’ if the general quality, as well as the DFR quality, was good. When the general study quality was good but only one DFR-criterion was fulfilled, studies were regarded as ‘moderate quality’. Studies lacking both DFR criteria, or without a good general quality assessment, were scored as ‘low quality’.
Data analysis
Extracted data were used to calculate DFR prevalence, defined as the proportion of patients achieving DFR, compared with those eligible for tapering medication. For each prevalence, the CI was calculated. Patients were considered eligible for tapering when they had achieved remission and subsequently were allowed to start tapering their medication. GCs were also considered as DMARDs. We specifically chose not to use the total study population as the denominator, because in some studies specific groups of patients were not allowed to taper their medication due to study protocol.
Sustained DFR (SDFR) was defined as the percentage of patients with a DFR duration of >12 months since DMARD stop, relative to the number of patients eligible for tapering. Reported flares were categorised and summarised according to the time period in which they occurred: (i) during tapering, (ii) in the first year after achieving DFR (‘early flares’) and (iii) after more than 1 year of DFR (‘late flares’). Results on DFR were summarised in a narrative overview, also in relation to study quality. Due to expected heterogeneity in study design and study populations, pooled effect estimates were not calculated.
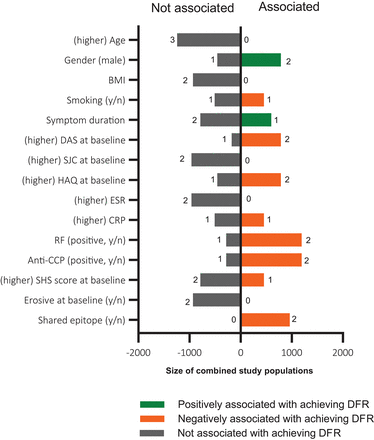
Additionally, the data were reviewed on potential predictors for achieving DFR. We used the same methods for data extraction and assessment as described for DFR prevalence. Predictors of DFR were summarised. Results on variables evaluated in more than one high-quality or moderate-quality article were graphically presented, based on statistical significance obtained with regression analysis. If univariate and multivariate analyses were both conducted, results of the multivariate analysis were used. For each predictor, the number of studies and the total number of patients within these studies were presented and the direction of the effect was indicated.
RESULTS
Study selection
Our search resulted in 631 articles, of which 51 articles were considered eligible for inclusion (figure 1). These 51 articles comprised data from 19 studies, 14 clinical trials and 5 observational cohorts.
Flow diagram of study selection. DMARDs, disease-modifying antirheumatic drugs.
Quality assessment
Both the quality of the study in general and the description of DFR were evaluated, resulting in a final quality rating. Eleven out of 14 clinical trials and two out of five observational cohorts showed a good general quality (table 1). Notably, the tapering methods were better described for clinical trials than for observational cohorts. Of the 13 studies with a good general quality, seven fulfilled both quality criteria for DFR and were regarded as high quality. These seven high-quality studies comprised five clinical trials and two observational cohorts. Of the remaining six studies, two studies were of moderate quality since only one DFR criterion was fulfilled. The four other studies did not fulfil any DFR quality criteria and were regarded low quality (table 1).
Assessment of general study quality and DFR quality, resulting in final categorization as high, moderate or low-quality study
Because of fundamental differences in study design, DFR prevalence and flare rates from clinical trials and observational cohorts were presented separately. Also, only high-quality or moderate-quality studies were presented in the result section. Nonetheless, all prevalence, including those of low-quality studies, can be found in table 2.
Prevalence of DMARD-free remission and flares
Clinical trials
Study characteristics
Study populations varied in RA classification (1987 vs 2010 criteria), disease stage/duration (early vs established) and disease activity (supplementary table S5). Overall, trials were performed in two ‘settings’: early, DMARD-naïve RA and established RA. Studies including early RA had a treat-to-target approach, and when remission was achieved, DMARDs were tapered. This was all conducted in a relative short period of time (n=7).13–19 The established RA studies (disease duration 3.1–11.3 years, n=6) either included patients with active disease who first changed DMARD treatment and subsequently became eligible for tapering (n=2)20 21 or selected patients who were in longstanding remission and were directly considered eligible for tapering (n=4).22–25 All established RA studies were of low quality, except 1 which was of moderate quality.18 One study, including patients in sustained remission, did not report disease duration.19
DMARD tapering
Tapering of DMARDs was initiated when patients fulfilled the study-specific eligibility criteria for tapering, in which some were stricter than others (supplementary table S5). Methods of tapering varied from immediate DMARD stop to one-by-one gradual tapering of DMARDs over the course of a year. In general, tapering of biologic DMARDs took place before tapering of conventional synthetic DMARDs. Flare rates during tapering ranged from 41.8% to 75.0% (table 2, figure 2).
Summary of flare rate and DFR prevalence, all as percentage of the number of patients that were eligible for DMARD tapering, depicted on a timeline. DFR prevalence was grouped by the duration of DFR. Data are presented as DFR percentage (CI). Data were based on high-quality or moderate-quality studies. Prevalence and CIs were calculated using the number of DFR patients divided by the number of patients eligible for tapering. Results from observational studies are indicated in italic. *indicates that studies that allowed the use of i.a. or systemic corticosteroids in patients that were considered to be in DFR(absolute number of patients that used corticosteroids after DMARD stop was not reported) or use in DMARD-free status was not clearly reported. X indicates moderate-quality studies. DMARD, disease-modifying antirheumatic drugs; DFR, DMARD-free remission; SDFR, sustained DMARD-free remission.
Definitions of DMARD-free remission
Overall, the remission criterion used to define DFR was mainly DAS44 or DAS28 remission. The DFR rates were either given as a point prevalence, thus at the moment of DMARD stop, or combined with a minimal DFR period of several months (table 2, figure 2). Nevertheless, most studies did not put much emphasis on a minimal duration of the drug-free state as a requirement to achieve DFR. Importantly, three studies that clearly defined DFR (two high-quality, one moderate-quality) allowed i.a. or oral GCs during DFR, without reporting the actual use.13 17 18
Prevalence of DMARD-free remission
In the five high-quality clinical trials, the reported prevalence of DFR (DFR <12 months) ranged from 5.0% to 24.3% (relative to the number of patients eligible for tapering). The two moderate-quality studies reported a DFR prevalence of 5.9% and 21.9% (table 2, figure 2), respectively. When studies that allowed GCs while being in DFR were excluded, DFR occurred in 5.0%–23.0%. SDFR (DFR >12 months) was only reported in two clinical trials and showed a prevalence of 11.6% and 19.4% (relative to patients eligible for tapering).
Evaluation of DFR prevalence, in high-quality and moderate-quality studies, in relation to the trial ‘settings’ was hampered by the fact that only one study was performed in established RA where DMARDs were tapered after prolonged remission,18 revealing a DFR prevalence of 5.9% compared with the prevalence of 5.0%–24.3% in studies that tapered DMARDs in early RA.13–17 32
Early flares (≤12 months after DMARD-stop) were reported in one high-quality study and occurred in 10.4% of patients eligible for tapering. Late flares (>12 months after DMARD-stop) were reported by another study and occurred in 3.5% of patients (table 2).
Observational cohorts
Study characteristics
Patients included in the observational cohorts were diagnosed between 1986 and 2011 (n=5). Patients in the observational cohorts were, compared with clinical trials, included in an earlier time period, but had a longer follow-up. Diagnosis was based on the 1987 criteria27 29 31 or expert opinion.28 30 Treatment was less protocolised compared with the clinical trials, and a treat-to-target approach was only used in three studies,27–29 of which two had a high quality (table 2).
DMARD tapering
Eligibility for tapering was only clearly reported in one study.28
Definitions of DMARD-free remission
Remission within DFR was defined as the absence of clinical synovitis (table 2), except for one study that used a DAS28 cut-off (DAS28<2.6).28 All five observational cohorts reported on SDFR (DFR >12 months), whereas one also reported on DFR after 6 months. In two studies, of which one was a high-quality study, i.a. and oral GC were allowed while being in DFR; the actual use was not reported.
Prevalence of DMARD-free remission
DFR prevalence (<12 months) was 23.6% of patients eligible for tapering and was reported in one high-quality study.28 The prevalence of SDFR ranged from 11.8% to 17.8% (relative to patients eligible for tapering)(table 2, Figure 2).27 28 If we exclude the studies that allowed GCs during DFR, one high-quality study remained with an SDFR prevalence of 17.8%.27 We did not compare DFR prevalence between studies that did and did not apply a treat-to-target approach, because all studies without a treat-to-target approach were of low quality.
Early flares (≤12 months after DMARD stop) were reported in one high-quality study and occurred in 11.8% of patients eligible for tapering. Late flares were reported by the other high-quality study and were seen in 0.3% of patients eligible for tapering (table 2).
Predictors of DFR
All factors that were analysed for their potential association with achieving DFR were evaluated (supplementary table S6). Due to heterogeneity in evaluated effect estimates, effect sizes could not be compared and meta-analyses not performed. For predictors that were studied in more than one high-quality or moderate-quality study, the association with achieving DFR is summarised in figure 3 (see also supplementary table S7). The figure includes information on the number of studies with/without an association with DFR, the total number of patients in these studies and the directionality of the effect (if present). The absence of autoantibodies and HLA-shared epitope alleles were predictive for achieving DFR. Many patient characteristics (eg, age, body mass index, swollen joint count (SJC), estimated sedimentation rate (ESR), erosions at baseline) were not associated with the chance of achieving DFR. For some, characteristic findings were inconsistent. Results on symptom duration, for example, showed ambiguous results (supplementary table S6/7).
Overview of studied predictors of achieving DMARD-free remission. Data are presented from variables that were reported in >1 study, based on statistical significance obtained in regression analysis. If both univariable and multivariable regression was applied, the result of the multivariable regression was used. Presented are the absence (left panel) and presence of an association with achieving DFR over time (right panel), the number of studies is indicated per predictor, the total number of patients in these studies is plotted on the x-axis. The directionality of the effect is indicated in colours, green indicates an increased risk of achieving DFR, red indicates a decreased risk of achieving DFR. For symptom duration, no differentiation was made for analyses using this as continuous or categorical variable. BMI, body mass index; CRP, C reactive protein; DAS, Disease Activity Score; ESR, estimated sedimentation rate; HAQ, Health Assessment Questionnaire; RF, rheumatoid factor; SJC, swollen joint count.
DISCUSSION
This systematic literature review was conducted in accordance with PRISMA guidelines and provides insight into the occurrence and sustainability of DFR in RA. The prevalence of DFR (DFR≤12 months) was 5.0%–24.3%,14–17 32 while SDFR (DFR>12 months) was achievable in 11.6%–19.4% of patients eligible for tapering.
Remission criteria used to define DFR varied widely, and the temporal aspect (sustainability) varied as well or was not reported. Moreover, in some studies, concomitant use of GC was allowed while patients were in DFR. This might falsely inflate DFR prevalence, but to what extent this occurred is unclear as actual use was not reported. Exclusion of aforementioned studies did not affect our results. To increase homogeneity, quality criteria were used, and final conclusions were only based on high-uality and moderate-quality studies, which resulted in a narrative overview of DFR prevalence (figure 2).
We observed different DFR prevalence depending on the duration of the DFR period. To allow a fair comparison of DFR prevalence, we categorised the duration of DFR in groups. SDFR was defined as a DMARD-free period >12 months. Higher prevalence was observed when DFR had a less stringent criterion for sustainability (figure 2). In line with this, flares occurred most often during tapering and in the first months after DMARD stop. This time effect underlines the relevance of defining sustainability of DFR in future studies.
DFR and SDFR might be fundamentally different. Short-term DFR might indicate that disease activity was suppressed, but not necessarily resolved, and could revive after the disappearance of suppressive treatment. Moreover, early flares (≤12 months after DFR) occur more often than late flares (>12 months after DFR), which might indicate that autoimmunity was not completely silenced. In our opinion, patients in SDFR (DFR>12 months) better resemble silencing of autoimmunity and may have achieved a proxy for cure. Therefore, SDFR can become an important outcome for clinical trials. Because late flares (often occurring years after DMARD-stop) might be pathophysiologically different from early flares, it is an interesting subject for future studies to explore the triggers or pathophysiologic mechanisms involved in late reactivation of the autoimmune process.
Notably, despite differences in study design, the DFR prevalence observed in observational cohorts and clinical trials was comparable. This supports the robustness of the observed frequencies. We were unable to investigate how long remission should be sustained before tapering can be initiated, because too few high-quality studies were performed in patients with established RA and longstanding remission. Additionally, due to an insufficient amount of studies, nothing can be said about the change of achieving DFR after treatment with certain conventional or biologic DMARDs.
We could not evaluate whether the method of tapering influenced the frequency of SDFR. It has been suggested that gradual tapering results in less flares compared with abrupt cessation.2 Also, the stringency of the remission criterion for initiation of tapering might be of influence, whereby less stringent criteria might increase the risk of flares. Evaluation of the methods of DMARD tapering was beyond the scope of this review, and a relevant subject for further studies as insight into the most effective tapering method may positively influence the chance to achieve SDFR. Another issue for further studies is the assessment of the likelihood to achieve remission for patients that flare after having been in DFR. From studies on patients that flared during tapering, it is known that the majority of patients achieve remission by restarting the same DMARD.33 Whether this is similar for patients that flare after DMARD stop is not yet systematically studied.
Studying the prevalence of DFR and predictors for DFR does not answer the question whether the absence of clinical signs and symptoms without treatment exhibited the natural course of RA in these patients,34 or was induced by DMARD treatment. This could not be answered within our SLR, nor could we compare studies for treatment intensity (eg, reflected by treat-to-target) due to the lack of high-quality studies without a treat-to-target approach. One high-quality study compared a treat-to-target approach that aimed for a DAS<1.6 with an approach that aimed for a DAS<2.4 and reported that patients achieved DFR more often when aimed for a DAS<1.6 (18% vs 8%, respectively), suggesting that intensive treatment is helpful in inducing DFR.35 However, the clinical trials rarely used DFR as a primary outcome, and, therefore, the question to what extent the frequency of DFR can be achieved by treatment remains a subject for future studies.
Although we tried to find predictors for DFR, it remains uncertain which patients are able to stop their DMARDs successfully. Meta-analyses could not be performed due to the heterogeneity of studies and effect estimates. Therefore, we summarised and graphically presented data on predictors using predefined criteria, but this methodology is far less optimal than meta-analysis. Several patient characteristics (eg, age, SJC, ESR and erosiveness) were not associated with a higher chance of achieving DFR. Results on symptom duration were conflicting, as the relation between DFR and symptom duration was non-significant, but showed a strong tendency towards significance in part of the studies. Furthermore, it is known that the association with DFR is not linear but refined to a short period of time30 (ie, the window of opportunity), and associations may remain undetected if symptom duration is analysed as a continuous variable. Absence of autoantibodies was the best predictor for DFR. Although effect sizes were not involved in our analyses, the absence of autoantibodies alone is not sufficient to accurately guide taper decisions in daily practice. Therefore, effective pursuit of SDFR in clinical practice requires more insight into subsets of patients that are likely to achieve SDFR.
Acknowledging the importance of the autoantibody status as predictor, the SDFR prevalence will be different for autoantibody-positive and autoantibody-negative patients. We could not stratify the results on SDFR prevalence for autoantibody status as the prevalence reported in the included cohorts and trials was not always stratified for autoantibodies. However, the studies that included information on autoantibody status in their patient characteristics reported that 52%–100% of patients were autoantibody positive (supplementary table S5).
Since conducting a thorough systematic literature review is time demanding, a time gap exists between the actual literature search (March 2019) and publication of the results. As a result, relevant articles in this time interval are not included. A non-systematic screening of articles published in this period revealed the BioRRA study,36 published in December 2019. This study focuses on predictors of flare after DMARD cessation and reported a 52% flare rate (DAS28-CRP≥2.4) after abrupt DMARD cessation. Predictors of flares were, among other things, absence of Boolean remission at baseline, RF positivity and IL-27. Biomarkers predictive of DFR, as identified in other recent studies, were calprotectin levels and several serum protein levels among which SAA.37 38 Calprotectin and SAA are both acute phase reactants. However, none of these markers were yet validated in independent studies.
From the patients’ perspective, achieving SDFR is beneficial; it was recently reported to be associated with normalisation of functional disability and resolution of symptoms, for example, fatigue.27 Unfortunately, clinical trials infrequently evaluated SDFR. If future trials would be designed with DFR/SDFR as primary outcome, consensus of the definition of remission and the duration of DMARD-free state is required to promote comparability of findings between studies. This may require OMERACT Initiatives.
In conclusion, DFR is achievable in RA and is sustainable in ~10%–20% of patients. DFR can become an important outcome measure for clinical trials and requires consistency in the definition. Considering the relative short follow-up after DMARD stop in current clinical trials and the high rate of flares in the first year after DMARD stop, we propose to incorporate a DMARD-free follow-up of at least 1 year, to ensure that DFR is sustainable.
Acknowledgments
We would like to thank J. Schoones, librarian of Leiden University Medical Centrum, for constructing and carrying out the literature search.
REFERENCES
Footnotes
MV and EV contributed equally
Contributors MV and EvM made a substantial contribution to the acquisition and analysis of the data. All authors made a substantial contribution to the interpretation of the data and the conception and design of the work. All authors approved the final version of the manuscript.
Funding The research leading to these results has received funding from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation program (starting grant, agreement no 714312) and from the Dutch Arthritis Foundation. The funding source had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
Competing interests None declared.
Patient consent for publication Not required.
Ethics approval Research ethics approval was not obtained since this was not applicable for this type of study.
Data sharing statement All data relevant to the study are included in the article or uploaded as supplementary information.
Provenance and peer review Not commissioned; externally peer reviewed.