Article Text
Abstract
Background Despite vitamin K antagonists (VKA) being the gold standard in the prevention of thromboembolic events in antiphospholipid syndrome (APS), non-vitamin K antagonists oral anticoagulants/direct oral anticoagulants (DOACs) have been used off-label.
Objective We aimed to perform a systematic review comparing DOACs to VKA regarding prevention of thromboembolic events, occurrence of bleeding events and mortality in patients with APS.
Methods An electronic database search was performed through MEDLINE, CENTRAL and Web of Science. After data extraction, we pooled the results using risk ratio (RR) and 95% CI. Heterogeneity was assessed using the I². The outcomes considered were all thromboembolic events as primary, and major bleeding, all bleeding events and mortality as secondary. Evidence confidence was assessed using the Grading of Recommendations Assessment, Development and Evaluation methodology.
Results We included 7 studies and a total of 835 patients for analyses. Thromboembolic events were significantly increased in DOACs arm, compared with VKA—RR 1.69, 95% CI 1.09 to 2.62, I²—24%, n=719, 6 studies. In studies using exclusively rivaroxaban, which was the most representative drug in all included studies, the thromboembolic risk was increased threefold (RR 3.36, 95% CI 1.53 to 7.37). The risks of major bleeding, all bleeding events and mortality were not significantly different from control arm. The grade of certainty of our results is very low.
Conclusions Current evidence suggests DOACs use, particularly rivaroxaban, among patients with APS, is less effective than VKA since it is associated with 69% increased risk of thromboembolic events.
Trial registration number CRD42020216178.
- antiphospholipid syndrome
- autoantibodies
- autoimmune diseases
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
Key messages
What is already known about this subject?
Direct oral anticoagulants (DOACs) have been used off-label in the primary and secondary prevention of thromboembolic events in antiphospholipid syndrome.
What does this study add?
This systematic review and meta-analysis shows that DOACs increase the relative risk of thromboembolic events, major bleeding events and mortality in these patients, compared with vitamin K antagonists.
How might this impact on clinical practice or further developments?
Current evidence does not support the use of DOACs, particularly rivaroxaban, in antiphospholipid syndrome. Thus, vitamin K antagonists should remain as gold standard in these patients.
Introduction
Antiphospholipid syndrome (APS) is an acquired autoimmune disease defined by the association of thromboembolic events (venous, arterial or microvascular) and/or pregnancy morbidity and the persistent presence of antiphospholipid (aPL) antibodies, such as lupus anticoagulant, anticardiolipin and anti-β2-glycoprotein 1.1 2 Triple-positive patients, who show a worse prognosis, represent less than 50% of those positive for one or two tests.3 A previous systematic review suggests that aPL antibodies were detected in 6% of women with pregnancy morbidity, in 13.5% of patients with stroke/transient ischaemic attack (TIA), 11% with myocardial infarction (MI) and 9.5% with deep vein thrombosis.4 Therefore, being thromboembolic diseases of major concern due to their high prevalence and often fatal consequences,5 the diagnosis and prognosis of APS should not be underestimated and treated accordingly.
Vitamin K antagonists (VKA) have been the gold standard in the primary and secondary prevention of thromboembolic events in APS. The target international normalised ratios (INR) interval should be between 2.0 and 3.0, but long-term treatment is a great medical challenge in these patients, particularly due to the risk of major bleeding.6 7
Another class of anticoagulants, the non-vitamin K antagonists oral anticoagulants (NOACs), also called direct oral anticoagulants (DOACs), have been used in many countries worldwide in the treatment and prevention of venous thromboembolism (VTE) as well as in stroke prevention in atrial fibrillation. DOACs include drugs such as apixaban, edoxaban, dabigatran and rivaroxaban.8 DOACs revealed several advantages over VKA: lower incidence of major bleeding, minor drug and food interactions, rapid onset (and also offset) of action, more predictable pharmacokinetics and pharmacodynamics and lack of need for laboratory monitoring with higher patients’ satisfaction.8–10
On the other hand, the data and the experience in this area are limited and heterogeneous increasing the uncertainty about the use of DOACs in APS.
Therefore, we aimed to perform a systematic review to compare DOACs to VKA regarding prevention of thromboembolic events, occurrence of bleeding events and mortality in patients with APS.
Methods
This systematic review followed the principles of MOOSE and PRISMA11 12 and was registered in PROSPERO: CRD42020216178.
Eligibility criteria
We considered published longitudinal studies (randomised controlled trials (RCTs) and observational studies, whether retrospective or prospective) comparing DOACs with VKA control group in adult patients diagnosed with APS. The type of APS (primary vs secondary), previously registered thromboembolic events (venous, arterial or microvascular) or the aPL antibodies profile were not initially relevant for the eligibility criteria. The outcomes considered were all thromboembolic events as primary, and major bleeding, all bleeding events and mortality as secondary. As rule, our all bleeding events encompass any type of bleeding, either major, clinically relevant non-major or minor.
Reviews, case series, case reports, commentaries or studies with unclear outcomes were not included.
Information sources and search strategies
An electronic database search for relevant material for inclusion criteria through MEDLINE, CENTRAL (Cochrane Central Register of Controlled Trials) and Web of Science was performed in March 2020. There were no restrictions on language or publication date.
The search strategy is detailed in online supplemental data 1.
Supplemental material
Studies records and data extraction
Two reviewers (NK and MA) screened the titles and abstracts yielded by the searches against the inclusion criteria and then read the full text reports and determined whether they met the inclusion criteria or not, the discrepancies being resolved by consensus. Reasons for the exclusion of articles were recorded at the full text screening stage. The data from included studies were uploaded onto a prepilot form, which included information such as study type, interventions, inclusion criteria, follow-up time, population main characteristics and outcomes. When studies presented different estimates, we used the most precise or adjusted measure.
Risk of bias
Each study was evaluated independently by two authors (NK and MA) in each of the domains of bias contained in the tools. For RCTs, we used the Cochrane risk of bias tool (RoB 2.0 tool)13 and for observational studies, the ROBINS-I tool,14 where all domains were classified accordingly to the algorithm. Then, the overall risk of bias judgement was performed. In RCTs, the final decision was one of: low risk, some concerns or high risk, while in observational studies it was one of: low risk, moderate risk, serious risk, critical risk or no information.
In case of posthoc analyses, while using data from the study, we additionally approached each one of the original trials, to access the risks of bias separately.
Data synthesis
The data was pooled using RevMan V.5.3 (The Nordic Cochrane Centre, Copenhagen; The Cochrane Collaboration, 2014).15
In accessing results, the intention-to-treat analysis was used, always favouring the bigger denominator.
The outcomes were treated as a dichotomous data and risk ratio (RR) and 95% CI were used to estimate pooled results from studies.15
Heterogeneity was assessed using the I2.16 The I2 statistics measures the percentage of total variation between studies attributed to interstudy heterogeneity rather than random. Statistical heterogeneity was considered substantial if I2 >50%. Fixed effects model was used by default because we wanted to estimate the mean effect of NOACs/DOACs in patients with APS. DerSimonian and Laird random effects model was only used if I2 > 50%.15 17 18
Publication bias assessment was performed through funnel plot examination if more than 10 studies were included.19
Subgroup analyses were done for type of study (RCT vs observational), accuracy/certainty of APS diagnosis (defined according to Sapporo criteria),1 triple-positive patients (<60% or≥60%) and type of DOACs used (just rivaroxaban vs other DOACs±rivaroxaban).
Assessment of confidence in cumulative evidence
As recommended by the Grading of Recommendations Assessment, Development and Evaluation (GRADE) Working Group methodology, two reviewers independently (NK and MA) assessed all the critical outcomes in the following domains: risk of bias, inconsistency, indirectness, imprecision and publication bias.20 21 The confidence on the pooled evidence was graded as very low, low, moderate or high. The pooled relative risks, as well as absolute risk measures, and the confidence on the pooled evidence were reported in the summary of findings table.15
Results
Included studies
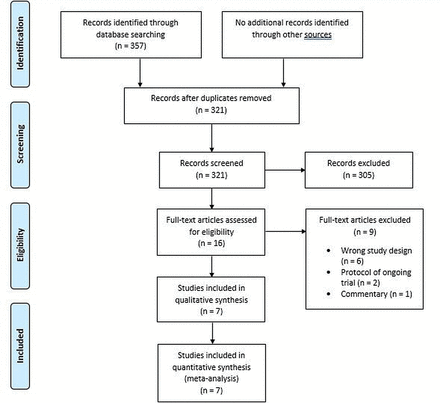
The search showed 357 uploaded references and after removing all duplicates, 321 remained. Then, 305 were excluded for one of the following reasons: background article, wrong study design (the model designed for these studies did not meet our inclusion criteria), wrong drug, wrong population, wrong outcome, duplicate; and just 16 full-text articles were assessed for eligibility, 6 of which had a wrong study design, 2 were protocols of ongoing trials and 1 was a commentary to Pengo et al22 (figure 1).
Study flow chart.
More detailed late stage/full text exclusion criteria are available in online supplemental data 2.
Finally, seven studies published between 2016 and 2019 were included in quantitative synthesis: three open-label RCTs;22–24 one posthoc APS subgroup analysis (Goldhaber et al25 of three RCTs (RE-COVER,26 RE-COVER II27 and RE-MEDY28 trials)) evaluating dabigatran in the treatment and prevention of VTE and three cohort studies (two prospective29 30 and one retrospective).31
Overall, this review included 835 patients with APS—not all studies were clear about using or not the revised Sapporo criteria1 in diagnosing APS—, followed for a period range between 210 days and 60 months. Out of these, 395 constituted the intervention arm (64.3% women) and 440 the control arm (64.3% women). The mean age was similar in both groups, 44.0–47.8 years range for DOACs and 42.6–51.0 years range for VKA. The study by Martinelli et al29 was not included, since authors only present patients’ age at first thromboembolic event.
Regarding DOACs, 265 patients were taking rivaroxaban, 75 dabigatran, 43 apixaban and 12 Edoxaban.
Most trials had warfarin as control, with exception of Ordi-Ros et al24 and Malec et al30 trials, which do not specify the VKA used.
All studies provided data for the primary and/or secondary outcomes of this review22–25 29–31 but only those with at least one event in each arm contributed for the meta-analysis.
Overall, there were 74 TE events (primary outcome) in the included trials, corresponding 55% to arterial and 45% to venous thrombosis. Out of 43 registered events in DOACs arm, 28 (65%) were arterial, largely driven by MI and stroke/TIA, 7 and 20 events, respectively (online supplemental data 3). In VKA arm, despite venous predominance, 13 (42%) in 31 events were arterial, out of which 1 corresponded to MI and 8 to stroke/TIA (online supplemental data 3).
More detailed characteristics of the included studies can be seen in table 1.
Summary of study characteristics
Risk of bias
Regarding all the outcomes, three studies22–24 were classified as having some concerns and four25 29–31 at serious/high risk of bias (tables 2 and 3).
Risk of bias assessment (RCTs)—TE events, major bleeding, all bleeding events and mortality
Risk of bias assessment (observational studies)—TE events, major bleeding, all bleeding events and mortality
Three RCTs22–24 offer some bias concerns due to deviation from intended intervention. Even though these trials were overseen by independent committees or had blinded end point adjudication, they were open label to ensure optimum drug dosing, monitoring and management.
The study by Goldhaber et al25 presents high risk due to selective reporting bias, since it was a posthoc analysis of three RCTs and there is no previously registered protocol available.
The study by Martinelli et al29 has serious risk of bias due to confounding—patients’ aPL profile was not appropriately controlled for.
The study by Malec et al30 has serious risk of bias due to confounding (risk factors as arterial hypertension, dyslipidaemia and triple APS positivity were not adjusted among arms), selection of participants (for the intervention arm authors only comprised patients who preferred a NOAC/DOAC or had unstable anticoagulation with VKAs, defined as time to therapeutic range below 50% within the previous 6 months, not being clear about the remaining patients), missing data (duration of follow-up differed between arms—mean 45 months vs 62 months) and measurement of the outcomes (TE events, major bleeding and all bleeding events outcomes were assessed by clinicians aware of the intervention received by study participants).
In addition, the study by Sato et al31 has serious risk of bias in the classification of interventions (drug regimen—dosage, rout of administration, frequency—is not well defined), measurement of outcomes and selection of reported results. Being a retrospective cohort, outcome assessors were aware of the interventions received.
Outcomes
Primary: TE events
Thromboembolic events were significantly increased in the DOACs arm, compared with the VKA arm—RR 1.69 95% CI 1.09 to 2.62; I²=24%; n=719; six studies (figure 2).
Forest plot of the pooled analysis comparing DOACs vs VKA regarding TE events, major bleeding, all bleeding events and mortality. *Random effect, I²>50%. DOACs, direct oral anticoagulants; TE, thromboembolism; VKA, vitamin K antagonists.
Secondary: major bleeding, all bleeding events and mortality
DOACs did not significantly increase the risk of major bleeding or mortality with RR 1.22 (95% CI 0.72 to 2.07; I²=0%; n=691; five studies) and RR 1.17 (95% CI 0.48 to 2.84; I²=0%; n=577; four studies), respectively (figure 2.
On the other hand, all bleeding events risk was non-significantly decreased in DOACs arm with RR 0.79 (95% CI 0.47 to 1.32; I²=66%; n=457; three studies) (figure 2).
Subgroup analysis
Although there was no statistical difference comparing RCTs to cohort studies, the magnitude of the increased risk of TE in the DOACs arm was superior in RCTs—RR 2.32, 95% CI 1.14 to 4.72; I²=49%; three studies (online supplemental data 4).
Regarding the certainty of APS diagnosis, there were no differences among the studies which mention the Sapporo criteria and the ones that do not (online supplemental data 4).
Studies including at least 60% of triple-positive patients presented higher risk of all bleeding events, compared with studies with <60% of triple positives, even though without achieving significant subgroup difference (RR 1.19 95% CI 0.77 to 1.85 vs RR 0.61 95% CI 0.41 to 0.90; p=0.03) (online supplemental data 4). There were no differences regarding other outcomes.
The exclusive use of rivaroxaban presented a statistically significant increased magnitude of effect regarding TE events (RR 3.36, 95% CI 1.53 to 7.37 vs RR 1.08, 95% CI 0.62 to 1.87; p=0.02) (online supplemental data 4).
Assessment of confidence in cumulative evidence
GRADE
Concerning TE events, the GRADE confidence is very low, being downgraded due to study design, risk of bias and imprecision (table 4). As for secondary outcomes, the GRADE confidence is also very low for the same reasons—except for all bleeding events, additionally downgraded due to indirectness (table 4).
Global grade summary of findings
Since we are combining RCTs with observational studies, the overall quality of evidence was assessed using the lowest quality of all included studies.
Separated GRADE analysis for RCTs and observational studies is available in online supplemental data 5. Considering only RCTs the GRADE is low, except for all bleeding events, which is very low.
Discussion
Our systematic review showed that the use of DOACs, compared with VKA, increased the relative risk of thromboembolic events by 69% in APS. In the DOACs arm, most of the events were arterial (MI and stroke/TIA)—65%, suggesting that patients with APS with history of arterial thrombosis or with other risk factors for arterial thrombosis may not be good candidates for DOACs, in particular for rivaroxaban. The analyses including studies with more robust methodology, namely RCTs and studies with high APS diagnostic certainty, presented an even higher risk of TE events in DOACs arm with RR 2.42 and RR 3.18, respectively. Additionally, in studies using exclusively rivaroxaban, which was the most representative drug in all included studies, the thromboembolic risk triples, when compared with VKA.
The risk of major bleeding or mortality was increased without achieving statistical significance. All bleeding events risk was non-significantly decreased in the DOACs arm. However, studies with higher risk patients (≥60% triple positive) showed quite the opposite. Despite non-significant results, this outcome increased substantially the risk comparing to VKA, and this probably is related to the worse thromboembolic and haemorrhagic profile of the included patients.3 Also, it is important to refer that in our population, a large portion of relevant bleeding events among female patients on rivaroxaban were heavy menstrual bleedings, being congruous with already existing data.32 33
Therefore, the results of this systematic review give scientific support to current recommendations for not recommending DOACs for secondary prevention of TE in patients with APS, VKA being the elected drug class in this context.6 7 Nevertheless, future data from observational studies and RCT will be important to clarify this risk/benefit in selected group of patients and different DOACs. For instance, ASTRO-APS trial with apixaban 5 mg two times a day compared with warfarin in patients with APS with VTE might change our present approach to this class of drugs.
In contrast to atrial fibrillation treatment, where VKA demonstrated to be less efficacious and safe,9 34 the reason behind DOACs failure in APS is still not consensual. Unlike VKA, they target only one coagulation factor, either Xa or IIa,35 and whether directed anticoagulation is sufficient or not in patients with APS remains unclear. Theoretically, and having in mind the pathophysiology of this syndrome, the presence of aPL antibodies constitutes one plausible justification since they can interfere with the normal pharmacokinetics of these drugs. Due to the fact that aPL antibodies increase lag time and time to peak thrombin generation23 and lead to platelet hyperactivation36 37 and fibrinolysis impairment,38 they might be responsible for DOACs’ resistance in APS. Other possible drawbacks are suboptimal drug concentration demonstrated in animal models,39 as well as the short drug half-life that may lead to a fast decline of anticoagulation effect and treatment failure if administrations are missed.40
Although meta-analyses on this topic have recently been published,35 41–43 our systematic review, in comparison, offers relevant advantages. Our focus was exclusively on patients with APS, without limiting TE events to either arterial, venous or microvascular. We included RCTs, which are known to better establish the causality between drugs and outcomes, and also observational studies, whose results provided data on all four existing DOACs and less strict APS population. Our pooled data also provide objective measure of the DOACs risk in APS as results achieved statistical significance concerning TE events, supporting some expert consensus.
There are some limitations regarding our review that should be taken into account. First, not all included studies were clear about using or not the revised Sapporo criteria1 in diagnosing APS. To overcome this limitation, we performed a subgroup analysis that did not show differences between studies with more or less restringing inclusion criteria. Second, approximately 67% of our population was on rivaroxaban, which could bias our conclusions. Indeed, in the subgroup analysis, in studies with heterogeneous use of DOACs the significant effect of TE events was lost. Third, the grade of certainty of our results is very low, due to methodological issues of the studies analysed. However, the inclusion of observational studies is important and offers some relevant advantages, such as a more diversified DOACs samples and the use of well-defined inclusion criteria, contributing for a more homogeneous population.
This is the best evidence available and until more robust evidence is published, physicians need to choose which drug benefits the most their patients based on this reality.
Currently, two more RCTs are ongoing: ASTRO-APS (apixaban for secondary prevention of TE among patients with APS) and RISAPS (rivaroxaban for patients who had stroke with APS, with or without SLE, follows the results of RAPS trial,23 with estimated completion dates for 2021 and 2023, respectively. The ASTRO-APS will include only a strict set of patients with APS with history of venous TE. In this study, patients with previous arterial thrombosis were excluded as these events may be a marker of higher thrombogenicity, recurrent events and potential DOACs treatment non-response.35 The RISAPS trial aims to study higher intensity anticoagulation with rivaroxaban (15 mg, two times a day, a dose recommended for the acute treatment of VTE) and warfarin (target INR 3.5)—these being the novelties of this study.
Notwithstanding our data, the ongoing trials, despite the existing differences in the population (stricter in ATRO-APS), will inform more robustly about the possible class effect of DOACs in APS. The need of further trials depends on these results.
In conclusion, our review suggests that DOACs use, particularly rivaroxaban, among patients with APS, appears to be less effective than VKA, since it is associated with increased risk of thromboembolic events. In fact, some authors44 45 report patients, mostly triple-positive, who experienced catastrophic APS—microthromboses involving at least three organs within a week46—after rivaroxaban introduction. Despite our results advising against DOACs, particularly rivaroxaban, judgements concerning other DOACs should be more watchful considering the ongoing trials, namely ASTRO-APS, that might provide additional data on this regard and consequently change the present approach to this class of drugs in patients with APS.
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information.
Ethics statements
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
Contributors NK and DC contributed to the concept and design of this review. NK, MA and DC contributed to data acquisition and data analysis. NK, MA, RP, AGA, JEF, JFF, FJP and DC contributed to interpretation of data, critically revised the manuscript and gave final approval of the submitted manuscript.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests None declared.
Provenance and peer review Not commissioned; externally peer reviewed.