Article Text
Abstract
Background Immune responses on SARS-CoV-2 vaccination in patients receiving anti-CD20 therapies are impaired but vary considerably. We conducted a systematic review and meta-analysis of the literature on SARS-CoV-2 vaccine induced humoral and cell-mediated immune response in patients previously treated with anti-CD20 antibodies.
Methods We searched PubMed, Embase, Medrxiv and SSRN using variations of search terms ‘anti-CD20’, ‘vaccine’ and ‘COVID’ and included original studies up to 21 August 2021. We excluded studies with missing data on humoral or cell-mediated immune response, unspecified methodology of response testing, unspecified timeframes between vaccination and blood sampling or low number of participants (≤3). We excluded individual patients with prior COVID-19 or incomplete vaccine courses. Primary endpoints were humoral and cell-mediated immune response rates. Subgroup analyses included time since anti-CD20 therapy, B cell depletion and indication for anti-CD20 therapy. We used random-effects models of proportions.
Findings Ninety studies were assessed. Inclusion criteria were met by 23 studies comprising 1342 patients. Overall rate of humoral response was 0.40 (95% CI 0.35 to 0.47). Overall rate of cell-mediated immune responses was 0.71 (95% CI 0.57 to 0.87). A time interval >6 months since last anti-CD20 therapy was associated with higher humoral response rates with 0.63 (95% CI 0.53 to 0.72) versus <6 months 0.2 (95% CI 0.03 to 0.43); p=0<01. Similarly, patients with circulating B cells more frequently showed humoral responses. Anti-CD20-treated kidney transplant recipients showed lower humoral response rates than patients with haematological malignancies or autoimmune disease.
Interpretation Patients on anti-CD20 therapies can develop humoral and cell-mediated immune responses after SARS-CoV-2 vaccination, but subgroups such as kidney transplant recipients or those with very recent therapy and depleted B cell are at high risk for non-seroconversion and should be individually assessed for personalised SARS-CoV-2 vaccination strategies. Potential limitations are small patient numbers and heterogeneity of studies included.
Funding This study was funded by Bern University Hospital.
- vaccination
- rituximab
- antirheumatic agents
- autoimmune diseases
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information. All relevant data are either available in manuscript or in the Supplementary Appendix. Raw data and code can be requested from the corresponding author.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
Key messages
What is already known about this subject?
Patients receiving anti-CD20 therapy show impaired immune responses to vaccines against different viral and bacterial pathogens.
Individual reports showed impairment of immune responses induced by SARS-CoV-2 vaccines after a very recent anti-CD20 therapy or in B cell depleted individuals.
What does this study add?
We synthesise the evidence for SARS-CoV-2 vaccine immunogenicity in this population over different disease populations and stratify according to different immunoassays, B cell depletion status and timing of anti-CD20 therapy.
How might this impact on clinical practice or further developments?
Our data can assist in the individual prediction of immune responses induced by SARS-CoV-2 vaccines for different subgroups of patients receiving B cell depleting therapy and guide strategies to optimise timing of vaccine and/or anti-CD20 therapy administration.
Introduction
The severe impact of the COVID-19 pandemic has led to the implementation of worldwide vaccination programmes. Even though SARS-CoV-2 vaccines have been made widely available, immunocompromised patients may still be at significant risk for severe COVID-19 after immunisation. B cell depleting therapy in particular is associated with impaired vaccination responses, as already demonstrated in prepandemic studies.1–3 In addition, disease entities and patient factors, such as individual and disease-specific B cell repopulation kinetics further influence response rates.4 5 Also, an adequate time interval between anti-CD20 therapy and vaccination seems crucial as previously demonstrated by immune response rates on influenza vaccines.4
With the broad availability of SARS-CoV-2 vaccines in many countries, strategies aimed at understanding and improving the immunogenicity of vaccines are urgently needed for patients undergoing anti-CD20 therapy. We therefore performed a systematic review and meta-analysis of humoral and cell-mediated immune responses after administration of SARS-CoV-2 vaccines in patients treated with anti-CD20 antibodies focusing on quantitative measures, diseases entities and duration since last anti-CD20 therapy.
Methods
We performed a systematic review and meta-analysis of peer-reviewed studies and preprints available online and reported it according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses.5
Definitions
We defined anti-CD20 therapy as treatment with rituximab, rituximab-abbs, rituximab-arrx, rituximab-hyaluronidase, rituximab-pvvr, ocrelizumab, obinutuzumab, ofatumumab and ibritumomab tiuxetan. We defined rituximab as monotherapy if explicitly reported. In most of the included studies, however, it was not defined if anti-CD20 treatment was administered as monotherapy or in combination with other immunosuppressives. In these cases, we assumed concomitant immunosuppressive comedication as disease types and enumerated baseline medication highly suggested anti-CD20 therapy being part of an immunosuppressive combination therapy.
We defined SARS-CoV-2 vaccine elicited humoral immune response as detection of antispike antibodies (anti-RBD or anti-S1 (spike protein) SARS-CoV-2) above the cut-off reported by the manufacturer of the given assay. Vaccine elicited cell-mediated immune response was defined as detection of SARS-CoV-2 specific T cells either measured by, T-EliSpot,6–9 interferon-γ release assays10 11 or activation-induced marker (AIM) detection12 13 in flow cytometry-sorted cells. AIM used for the detection of vaccine elicited T cells response were CD4 +CXCR5+PD1+and CD38+HLA-DR+12 as well as S-specific OX40 +41-BB+CD4+ and CD69+41BB+CD8+.13
Autoimmune diseases were defined as a collective of diseases characterised by aberrant immune responses including the presence of antibodies or T cells reacting with self-antigens that are treated with immunosuppressants.
Eligibility criteria
We considered all original research studies that investigated serological and/or cell-mediated responses on SARS-CoV-2 vaccination in patients with anti-CD20 therapies potentially eligible for inclusion. Prespecified exclusion criteria were exclusive focus on participants with previous COVID-19 infection or incomplete vaccination schedules, unspecified time frames between vaccination and blood sampling, unspecified methodology for detection of antibody-mediated or cell-mediated immunity (specification of manufactures and detection kits mandatory), number of investigated participants lower or equal than 3, missing numbers of positive versus negative humoral or cell-mediated immune responders. In addition, review and guideline articles as well as all search results not meeting the topic of our research question were excluded.
Information sources and search strategy
PubMed (up to 21 August 2021), Embase (up to 21 August 2021), as well as preprint servers medrxiv, SSRN and SSRN-Lancet (1 January 2020–21 August 2021) were accessed online and searched within title/abstract without language restrictions.
For PubMed,14 a search for “rituximab OR anti-cd20 AND covid AND vaccine” within title/abstract was performed. For Embase,15 a search for “rituximab OR anti-CD20 AND covid AND vaccine” within title/abstract was performed. For medrxiv,16 a search for “rituximab AND covid AND vaccine” was performed. Additionally, a search for “anti-CD20 AND covid AND vaccine” was performed. A third search using “anti-CD20 AND covid AND vaccine” was performed. For SSRN,17 a search for “rituximab AND covid AND vaccine” was performed. Another search using terms “anti-cd20 AND covid AND vaccine” was performed. For SSRN Preprints with The Lancet,18 a search for “rituximab AND covid AND vaccine” was performed. Another search for “anti-CD20 AND covid AND vaccine” was performed.
Selection process
We executed the process of studies selection in accordance with Cochrane recommendations.19 Two authors (SS and MA) independently assessed all search results of PubMed database, and two authors (SS and MBM) independently assessed all search results of EMBASE database and preprint servers. In cases of divergent selections, a third author (MA or MBM, respectively) was consulted. Fulfilments of inclusion and exclusion criteria were reviewed again by each of the three authors and decision processes were mutually rechecked and discussed thereafter. Discrepancies could be unequivocally resolved in all cases with full agreement by all authors. We did not need to apply the prespecified mode of majority decisions due to persistent disagreements. We did not apply automation tools.
Data collection process
Tabular and text data of study population subsets with a history of anti-CD20 therapies were manually copied and independently downloaded by each reviewer. Extraction of graphical figure data was performed by image analysis in selected cases. We did not apply automation tools.
Data items
Next to the inclusion and exclusion criteria specified previously, we extracted the following information from the searched studies:
Primary outcome data
Percentage of participants (with anti-CD20 therapies, no history of previous COVID-19 disease and complete SARS-CoV-2 vaccination course) with positive serological and/or cell-mediated immune response after SARS-CoV-2-vaccination.
Data for subgroup generation
Disease types of study population, type of immunosuppressive therapy (anti-CD20±other immunosuppressive treatment), immune assay types used and primary outcome data separated by time since last anti-CD20 treatment (before vs after 6 months). An exploratory post hoc analysis was made according to B cell depletion (‘yes’ vs ‘no’ as defined by the studies).
Data for quality evaluation
Study design, method of cell-mediated immune (CMI) response measurement, manufactures of detection kits and respective cut-off values for test positivity, title of study, digital object identifier and PubMed identifier for repeated duplication checks. In cases where cut-off values of a manufacturer’s kit for antibody or CMI response testing were not specified in the methods section, we retrieved these data from the manufacturer’s websites.
Risk of bias assessment
We manually assessed the risk of bias of included studies using the Newcastle-Ottawa Scale for assessing the quality of non-randomised studies in meta-analyses by Wells et al.20 Three investigators (SS, AB and MBM) independently assigned a number of quality criteria (minimum 0, maximum 9) for each study. A fourth investigator (MA) summarised results according to a prespecified mode of majority decision. Threshold of an optimal follow-up period after the second vaccine was estimated as at least 4 weeks after completed vaccination.21
Effect measures
For both outcomes of humoral and cell-mediated immunity, number and proportion of responders was used in the synthesis and presentation of results.
Synthesis methods
Synthesis was first obtained by tabulating the studies using Microsoft Excel and comparing against a list of exclusion criteria. No missing data were present in the included studies. We performed a random-effects meta-analysis of proportions using the method of Der Simonian & Laird, with pooled estimates calculated by Freeman-Tukey Double Arcsine Transformation22 to stabilise the variances. Statistical heterogeneity was quantified using the I2 measure, taken from the inverse-variance fixed-effect model.23 For individual studies, the Wilson score 95% CI is displayed. To explore possible causes of heterogeneity between studies, we performed prespecified subgroup analyses specified previously.
Quantitative analyses and graphical displays were done in Stata V.17 using the command metaprop V.10.1. Small study effects analysis was conducted in R V.4.0.5 (meta-package 4.19–1). No sensitivity analyses were performed.
Reporting bias assessment
Small study effects were assessed by a funnel plot and a regression test for funnel-plot asymmetry.24
Certainty assessment
No procedures were performed to assess confidence in the body of evidence.
Results
Search results
The study selection process is presented in detail in figure 1. Searches in PubMed14 and Embase (12) yielded 73 and 39 results, respectively. Searches within preprint servers yielded 12 studies from medrxiv16 and 23 studies from SSRN17 and SSRN Preprints with The Lancet.18 After removal of 57 duplicates, 90 studies remained for eligibility assessment. Title and abstract screening led to exclusion of 56 additional articles. Full-text screening of the remaining 34 articles then led to further exclusion of 11 articles (online supplemental appendix, pages 4–9). A total of 23 studies with data of 1342 participants fully met inclusion criteria and were eventually included in the meta-analysis.
Supplemental material
Flow chart describing the study search and selection process.
Study characteristics
Study characteristics of the included publications6–13 25–39 are summarised in table 1. Information to assay details of antibody detection and CMI are indicated in online supplemental appendix, pp. 9–10.
List of included studies
Risk of bias
Online supplemental appendix, p. 11 shows the results of the risk of bias assessment for the individual studies included. Applying the NOS eight out of nine possible quality rating criteria could be met (‘Adequacy of follow-up’ not applicable). Risk of bias ratings for the 23 included studies were low to moderate.
Results of individual studies
Details regarding the calculations of proportions for meta-analysis using a random-effects model calculation of the effect size are mentioned in the Methods section. Pooled estimates of proportions are shown, with 95% CI. The last column shows the weight of the specific study in percentage.
Humoral response to vaccination
Humoral responses were highly heterogeneous with a rate of responders ranging from 0 to about 80%, resulting in an overall humoral response rate of 40%. An I2 of 74% confirmed heterogeneity in this study collection (figure 2). Therefore, studies were subjected to prespecified subgroup analyses including time since last anti-CD20 therapy and anti-CD20 treatment indication.
Humoral immune responses across all included studies. Prop., proportion.
Stratification based on a 6 months threshold since last anti-CD20 therapy showed that studies with shorter intervals reported significantly lower numbers of responders (20% vs 63%) with exception for one study including exclusively patients with rheumatoid arthritis (figure 3). Benucci et al11 reported a markedly higher humoral response rate of 75% compared with other short-term studies, which probably accounts for the high heterogeneity of this subgroup.
Humoral immune responses according to prespecified subgroups of <6 or >6 months of time since the last dose of anti-CD20 therapy. Prop., proportion.
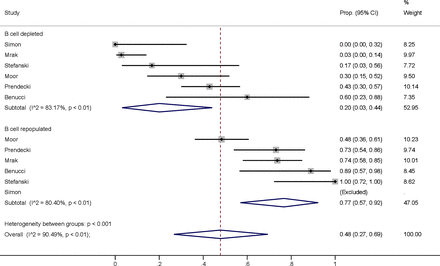
Similarly to the time threshold since last anti-CD20 therapy, we determined in an exploratory analysis whether current status of B cell depletion is associated with worse seroconversion rates. Patients with depleted B cells showed lower numbers of responders (20% vs 77%) (figure 4).
Humoral immune responses stratified by subgroups of patients with depleted versus replete B cell counts. Prop., proportion.
An analysis of humoral response by indication for B cell depletion (autoimmune diseases, haematological malignancy and kidney transplantation) is shown in figure 5. Pooled humoral response rates were similar for autoimmune diseases and cancer (43% vs 36%) but markedly lower for patients having undergone kidney transplantation (14%).
Humoral immune responses according to prespecified subgroups of indications for anti-CD20 therapy. Prop., proportion.
Online supplemental appendix p.2 shows humoral response stratified by types of immunoassay. For some assays, several studies were available. Other assays were used in a single study only or were even used in a combination within other assays within a single study study. Humoral response (pooled response rate) was overall comparable between studies using the Elecsys (Roche) assay, the Quant II (Abbott) assay and the Euroimmun assay. Benucci et al11 was an outlier as it was the only study using a ThermoFisher assay and yielded higher seroconversion rates as mentioned previously.
Cell-mediated immune response to vaccination
Cell-mediated immune response rates (not stratified, including EliSpot, IGRA and AIM) varied from 44% to 100%, with an overall pooled response rate of 71% (figure 6). The heterogeneity of the included studies is large as indicated by an I2 of 81.36%.
Cell-mediated immune responses across all included studies. Prop., proportion.
To address this, we next stratified response rates by assay type (online supplemental appendix p.3). EliSpot showed a mean response rate of 72%, compared with only 47% in IGRA. However, for IGRA, results from only two studies were available, which differed in CMI response rates (44 vs 100%) and in patient numbers (93 vs 4). AIM analysis showed a pooled positive response rate of 83%.
Sensitivity analyses and reporting bias
No sensitivity analyses were conducted. There was no indication of small study effects in the Funnel plot (p value of test for asymmetry: 0.638) (figure 7).
Funnel plot of all included studies.
Discussion
The present work provides an overview of seroconversion rates and cell-mediated immune responses after SARS-CoV-2 vaccines from the first two dozens of studies of patients with a history of anti-CD20 therapies. Currently, no systematic reviews are available for this topic.
Our analysis suggests that a remarkable heterogeneity in immunogenicity of SARS-CoV-2 vaccines exists, which partly results from differences in treatment indications, that is, underlying disease settings.
A key result is our finding that current B cell depletion and time since last anti-CD20 therapy administration severely impacts seroconversion rates. This is congruent with the results of individual studies of SARS-CoV-2 vaccines9 10 26 38 and has been found for influenza seroconversion in anti-CD20 therapies.4 This finding may be of particular interest for ideal scheduling of vaccination. However, we have previously reported that some SARS-CoV-2 vaccine-induced seroconversion rates can occur in patients with high CD4-positive T cell counts even at complete B cell depletion due to recent anti-CD20 therapy.10 Furthermore, we found that the different assays used in studies of cell-mediated immunity led to heterogeneous results. This highlights a general difference between quantitative IGRA and the more semiquantitative EliSpot analysis, that is, whenever an EliSpot yields a result comparable between patients and healthy controls, only the fraction of activated cells but not their quantitative activation is captured. Similar to our observation, EliSpot was reported to be more sensitive for diagnosing tuberculosis than quantitative IGRA40 with an unexplained discordance between the two assay types. Moreover, we report that the type of patient collective heavily influenced the observed immune responses against SARS-CoV-2.
The evidence base included in this review contains some limitations. First, some participants may have been included who had asymptomatic COVID-19, which was not detected by serological testing, for example, by antinucleocapsid immunoassays, and only 8 out of 23 included studies used an objective assessment of COVID-19 exposure such as prevaccination anti-S or presence of antinucleocapsid antibodies. Second, the seroconversion itself is a somewhat arbitrary outcome, which is heterogeneous due to manufacturer cut-offs, and no clear threshold for protective antibody levels exist to date. Furthermore, the seroconversion may not ultimately translate to protection from severe COVID-19 or symptomatic COVID-19 directly in patients with a history of anti-CD20 therapies. Therefore, the scarce available data on cell-mediated immunity was included in the present analysis, which represents a second although assay-dependent measure of immunity against SARS-CoV-2.
Published information was further insufficient to allow analysis of different anti-CD20 drugs or a better discrimination according to time since last anti-CD20 therapy or last vaccine as a continuous variable that may contribute to heterogeneity of the findings. Finally, for a closer stratification according to individual autoimmune diseases, the current population-level data were insufficient. For such investigations, access to patient-level data is required for analysis in future studies.
The present review process was further limited by an arbitrary timing of the literature search (21 August 2021) of a rapidly emerging knowledge database, which renders the current evidence preliminary rather than definitive and mandates additional meta-analyses in the future. Furthermore, no external experts in the field were consulted, and no unpublished studies or clinical study registry data were queried. Some potential sources of heterogeneity in seroconversion rates in the study population were not captured in subgroup analyses, such as the immunosuppressive comedication10 and potentially circulating CD4-positive T cell counts in patients on anti-CD20 therapy10 similar as in HIV studies.41 Finally, we did not perform an analysis of seroconversion rates according to the different vaccines administered, as population-level data did not sufficiently discriminate between vaccine types. Such analyses and are warranted in further studies of the evidence base.
Summary and clinical implications
The present analysis establishes implications for clinical practice and future research despite the heterogeneity of included studies. Patients with experience of anti-CD20 therapies that are vaccinated against SARS-CoV-2 mount humoral and cell-mediated immune responses to SARS-CoV-2 in 41% and 73% of patients, respectively, after predominantly two-dose regimen of vaccine. Patients with a treatment history of anti-CD20 therapies should be individually assessed for a personalised vaccination strategy against SARS-CoV-2. While the immunogenicity of additional vaccine doses against SARS-CoV-2 remains to be determined, we recommend a close assessment of vaccine-induced seroconversion in patients on anti-CD20 therapy for consideration of additional doses of SARS-CoV-2 vaccine. This is most crucial in those within 6 months since the last dose of anti-CD20 therapy, in those with currently low circulating B cell counts and in transplant recipients treated with multiple immunosuppressive comedications that all showed lower vaccine-driven immunogenicity. Persisting seronegativity after vaccination must be communicated to patients because of the potential eligibility for prophylactic treatments, or for therapeutic antibodies in case of SARS-CoV-2 infection where a mortality reduction was reported in seronegative patients.42 Furthermore, modulating or delaying immunosuppressive therapies for the sake of vaccine efficacy cannot currently be recommended based on the available data and would have to be carefully weighed against the risk of disease flare-up. Finally, future studies assessing SARS-CoV-2 vaccine efficacy in this population should determine the risk of breakthrough infections.
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information. All relevant data are either available in manuscript or in the Supplementary Appendix. Raw data and code can be requested from the corresponding author.
Ethics statements
Patient consent for publication
Ethics approval
This study does not involve human participants but reanalyses published data.
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
SS and MA are joint first authors.
Twitter @Andereggman, @NephMoor
Contributors MBM and DS conceived the study. SS, MA and MBM screened and selected studies. AL performed statistical analysis. SS, AB, MA and MBM assessed risk of bias. SS, MA, BM, MPH, AB, BM, CH, DS and MBM interpreted the data. SS, MA and MBM wrote the manuscript. MBM is the author responsible for the overall content as the guarantor. All authors critically reviewed and approved the final manuscript.
Funding The present study was funded by Bern University Hospital.
Disclaimer The funder had no role in the design, execution of the study, analysis of the data nor decision to submit the manuscript for publication.
Competing interests None declared.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.