Abstract
Sodium–glucose cotransporter 2 (SGLT2) inhibitors belong to a novel class of glucose-lowering medications that reduce plasma glucose concentrations by inhibiting glucose reabsorption by the kidney, inducing glucosuria. Their actions encompass reductions in HbA1c, fasting and postprandial blood glucose levels, body weight and BP. To date, empagliflozin and canagliflozin have additionally been shown to improve cardiovascular outcomes in high-risk individuals and to slow the progression of diabetic kidney disease. Adverse effects associated with this class include urinary frequency, dehydration, genitourinary tract infections and, rarely, euglycaemic diabetic ketoacidosis. Of the SGLT2 inhibitors, only canagliflozin has been linked to a higher risk of lower-extremity amputations and bone fractures compared with placebo. Optimal prescribing of agents within this relatively new drug category requires a full understanding of their risks in addition to their benefits.
Similar content being viewed by others
Introduction
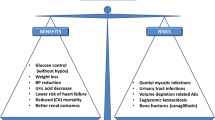
Sodium–glucose cotransporter 2 (SGLT2) inhibitors belong to a novel class of medications that reduce plasma glucose concentrations by increasing urinary glucose excretion. Members of this class approved for use in the USA and Europe are canagliflozin, dapagliflozin, empagliflozin and ertugliflozin. Besides decreasing plasma glucose, they also induce some weight loss, lower BP and carry a low risk of hypoglycaemia. Some SGLT2 inhibitors also have cardiovascular and renal benefits. They are generally well tolerated but have several well-recognised adverse effects that should be considered to optimise their risk to benefit ratio. The benefits vs risks of SGLT2 inhibitors are shown in Fig. 1.
Recognised major risks and benefits of SGLT2 inhibitors. To date, aonly empagliflozin and canagliflozin have demonstrated cardiovascular and renal benefits, and bincreased risk of amputations and fractures has only been observed with canagliflozin in large, randomised, controlled clinical trials. GU, genitourinary; HF, heart failure. This figure is available as part of a downloadable slideset
Beneficial effects of SGLT2 inhibitors
Glucose lowering
SGLT2 inhibitors have been studied as monotherapy or in combination with other oral agents or insulin for the management of hyperglycaemia in type 2 diabetes. SGLT2 inhibitors achieve a reduction in HbA1c of 4.4–12.1 mmol/mol (0.4–1.1%), depending on the baseline HbA1c and the specific drug and dose used [1,2,3,4]. HbA1c is reduced to a slightly greater extent by high-dose canagliflozin (vs other SGLT2 inhibitors), probably as a result of its additional action of inhibiting SGLT1 in the intestine. When compared head-to-head with dipeptidyl peptidase-4 (DPP-4) inhibitors, SGLT2 inhibitors appear minimally more potent [5]. Interestingly, compared with sulfonylureas, the initial HbA1c reduction is more prominent with sulfonylureas but there is partial loss of efficacy over time, resulting in a slight HbA1c advantage for SGLT2 inhibitors at 2 years [6]. SGLT2 inhibitors are associated with a low incidence of hypoglycaemia [1,2,3,4] and can be added to any existing diabetes treatment to effect a reduction in HbA1c regardless of background therapy.
Weight loss
Glucosuria-induced energy loss caused by SGLT2 inhibitors leads to weight loss, which appears to be sustained over time. The degree of weight loss varies slightly according to the agent and the dose used. A meta-analysis of randomised controlled trials involving participants treated with canagliflozin 300 mg, empagliflozin 25 mg or dapagliflozin 10 mg daily showed a weight loss of 2.66 kg, 1.81 kg and 1.80 kg, respectively, compared with placebo [7].
BP reduction
SGLT2 inhibitors lower BP by promoting osmotic diuresis and intravascular volume contraction. This effect does not appear to be linked to reduction in HbA1c, as individuals with moderately reduced renal function display decreased BP despite minimal HbA1c reduction. SGLT2 inhibitors decrease systolic BP by 3.4–5.4 mmHg and diastolic BP by 1.5–2.2 mmHg. Type 2 diabetic individuals with uncontrolled BP at baseline experience the largest reduction in systolic BP after SGLT2 inhibitor treatment [1,2,3,4].
Cardiovascular benefits
Among the SGLT2 inhibitors, empagliflozin and canagliflozin have been shown to significantly reduce cardiovascular events in individuals with underlying cardiovascular disease (CVD).
In the Empagliflozin, Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients (EMPA-REG OUTCOME) trial, involving individuals with diabetes and established CVD, participants randomised to receive empagliflozin displayed a lower rate of the primary major adverse cardiac events (MACE) outcomes compared with those who received placebo (HR 0.86 [95% CI 0.74, 0.99]; p = 0.04) [8]. This was predominantly driven by a 38% reduction in the risk of cardiovascular death; there was no significant between-group difference in the rates of myocardial infarction or stroke. Risk of death from any cause was reduced by 32% and risk of hospitalisation for heart failure was reduced by 35%. The difference in blood glucose control between the empagliflozin and placebo groups was small, suggesting that extra-glycaemic effects were responsible for the CVD outcome. In a post hoc analysis, the change in haematocrit (~3% absolute increase), corresponding to ~7% reduction in plasma volume, explained about 50% of the benefit of the drug on cardiovascular death [9]. Other mechanistic theories include a small increase in β-hydroxybutyrate, which may provide a more efficient energy source for the diabetic heart [10], and inhibition of the Na+/H+ exchanger in cardiac myocytes, which could improve energy dynamics within the heart [11].
The proportion of individuals experiencing stroke in the empagliflozin vs placebo group in the EMPA-REG OUTCOME study was 3.5% (164/4687) vs 3.0% (69/2333) (HR 1.18 [95% CI 0.89, 1.56]; p = 0.26) [8]. In a subsequent analysis, the investigators showed that this difference was driven by non-fatal ischaemic stroke, with no isolated increase in any specific subtype [12]. Importantly, the difference was due to events that occurred >90 days after the last intake of study drug, suggesting no direct effect of the drug. For stroke occurring during treatment or ≤ 90 days after the last dose of drug, the HR for empagliflozin vs placebo was 1.08 (95% CI 0.81, 1.45; p = 0.60).
In the Canagliflozin Cardiovascular Assessment Study (CANVAS) Program involving individuals at high cardiovascular risk, the primary MACE outcome was significantly reduced in the canagliflozin arm vs placebo arm (HR 0.86 [95% CI 0.75, 0.97]; p = 0.02 for superiority) [13]. None of the individual components of MACE, nor all-cause mortality, were significantly reduced by canagliflozin, but the drug did exhibit a similar benefit to empagliflozin (vs placebo) in reducing hospitalisations due to heart failure (HR 0.67 [95% CI 0.52, 0.87]). CANVAS recruited individuals with and without CVD, which might initially suggest potential benefits of canagliflozin in primary prevention [14]. However, the point estimate for the primary outcome was only 0.98 in those without established CVD, implying that the benefit may be mostly in secondary prevention. In contrast, the point estimate for heart failure hospitalisation was similar in both cohorts, suggesting that this specific cardiac benefit may extend to diabetic individuals without overt CVD.
Several observational studies have confirmed that SGLT2 inhibitors are associated with decreased risks of cardiovascular mortality and MACE and lower rates of hospitalisation for heart failure [15, 16]. The decrease in cardiovascular mortality and MACE occurs in individuals with and without CVD at baseline, suggesting that the benefits obtained with empagliflozin and canagliflozin in randomised trials in individuals at high cardiovascular risk may be a class effect applicable to a broad population of individuals with type 2 diabetes.
Reduction in nephropathy
The EMPA-REG OUTCOME trial studied the long-term renal effects of empagliflozin. The composite renal outcome included incident or worsening nephropathy (progression to macroalbuminuria, doubling of serum creatinine level, initiation of renal-replacement therapy, death from renal disease). Empagliflozin was associated with slower progression of kidney disease and lower rates of clinically relevant renal events than was placebo when added to standard care. Incident or worsening nephropathy occurred in 12.7% of participants in the empagliflozin group vs 18.8% in the placebo group (HR 0.61 [95% CI 0.53, 0.70]; p < 0.001). There was no significant between-group difference in the rate of incident albuminuria. The renal benefit was seen irrespective of baseline eGFR, occurring in individuals with an eGFR down to 30 ml min−1 [1.73 m]−2. The adverse-event profile of empagliflozin in individuals with impaired kidney function at baseline was similar to that reported in the overall trial population [17]. The mechanism behind the positive renal outcomes of empagliflozin probably includes a direct renovascular action. The short duration of the study and the modest decrease in HbA1c make it unlikely that the improved renal outcomes of this medication can be explained by its glucose-lowering effect.
In the CANVAS Program (which included CANVAS and CANVAS-Renal [CANVAS-R] studies, the latter study assessing specifically the effects on albuminuria), canagliflozin was found to have a beneficial effect on the progression of albuminuria (HR 0.73 [95% CI 0.67, 0.79]) and the composite outcome of a sustained 40% reduction in the eGFR, the need for renal-replacement therapy or death from renal causes (HR 0.60 [95% CI 0.47, 0.77]) [13]. Whether other SGLT2 inhibitors have similar beneficial renal effects is unknown but this would be expected based on their known mechanism of action.
Adverse effects of SGLT2 inhibitors
Genitourinary infections
The most common side effect of SGLT2 inhibitors is an increased incidence of genital mycotic infections (mainly balanitis and vulvovaginitis). Some, but not all, studies showed that these drugs are also associated with a small increase in the risk of urinary tract infections (UTIs) [8, 13, 18]. These effects are attributed to the increased glucosuria induced by these agents. Generally, the events are mild to moderate in severity and do not necessitate discontinuation of the drug. Infections of the upper urinary tract, such as pyelonephritis, and urosepsis are extremely rare complications.
A meta-analysis of randomised trials comparing SGLT2 inhibitors with placebo or other medication for type 2 diabetes showed that SGLT2 inhibitors were significantly associated with a fivefold increase in the risk of genital mycotic infections (OR 5.06 [95% CI 3.44, 7.45]) and a more modest increase in UTIs (OR 1.42 [95% CI 1.06, 1.90]) [18]. Genital mycotic infections can be treated with a single dose of an oral antifungal drug (e.g. fluconazole) or several days’ application of an antifungal cream (e.g. miconazole, clotrimazole). Uncomplicated UTIs can usually be managed with routine therapy. There are no data to support surveillance urinalyses or urine cultures in diabetic individuals taking SGLT2 inhibitors.
Diabetic ketoacidosis
Diabetic ketoacidosis (DKA) is a complication of treatment with SGLT2 inhibitors. It has sometimes been associated with serum glucose levels <13.9 mmol/l and has arguably been referred to as ‘euglycaemic DKA’. This occurs more frequently in individuals with type 1 diabetes treated off-label with SGLT2 inhibitors but can also occur in individuals with type 2 diabetes [19,20,21].
A recent analysis, using a large database of commercially insured individuals in the USA, compared a cohort of diabetic individuals who had newly started treatment with an SGLT2 inhibitor vs a DPP-4 inhibitor [21]. The unadjusted rate of DKA within 180 days after the initiation of an SGLT2 inhibitor was about twice the rate after the initiation of a DPP-4 inhibitor before propensity-score matching to adjust for differences in individuals’ characteristics (4.9 vs 2.3 events per 1000 person-years; HR 2.1 [95% CI 1.5, 2.9]). The same was true after propensity matching (HR 2.2 [95% CI 1.4, 3.6]).
In the EMPA-REG OUTCOME study the proportion of individuals with DKA was very low in both the empagliflozin and placebo groups (4/4687 [0.09%] vs 1/2333 [0.04%], respectively) and not statistically significantly different [8]. In the CANVAS Program, a small number of DKA events were observed in the canagliflozin and placebo groups (0.6 vs 0.3 events per 1000 patient-years, respectively; HR 2.33 [95% CI 0.76, 7.17]) [13].
Individuals presenting with euglycaemic DKA complain of nausea, vomiting and malaise. Biochemical evaluation reveals an anion-gap metabolic acidosis and positive serum and urine ketones. As mentioned, serum glucose is often lower than in diabetic individuals with traditional DKA because of increased renal clearance of glucose mediated by the drug. The absence of markedly elevated serum glucose levels can, however, delay recognition by both the affected individual and the clinician. Treatment of DKA includes administration of intravenous fluids, insulin, and monitoring and replacing the electrolytes. If a diabetic individual develops DKA during SGLT2 inhibitor therapy, the inhibitor should not be restarted, at least not immediately, as there have been several reports of individuals who have had recurrences of DKA with continuous SGLT2 inhibitor therapy [19]. It is reasonable in such cases to avoid use of the class going forward. SGLT2 inhibitor therapy should be also stopped during acute illness and at least 48 h before any planned surgical procedure, so that a catabolic state is not aggravated and the risk of DKA is minimised.
The mechanism of euglycaemic DKA has not been fully elucidated. Illness, surgical stress or ethanol binge, with a reduction in food intake and/or insulin doses, preceded the development of DKA in some cases. The significant improvement in hyperglycaemia following initiation of SGLT2 inhibitor treatment can lead to a decrease in insulin requirements in some individuals. The reduced insulin doses and increased insulin resistance caused by an acute illness may tip the balance towards ketosis [19]. In addition, SGLT2 inhibitors are associated with an increase in plasma glucagon levels through uncertain mechanisms [22, 23]. Hyperglucagonaemia increases the tendency towards ketone production at the level of the liver [24]. By promoting osmotic diuresis, SGLT2 inhibitors also predispose to dehydration and urinary loss of sodium. This may worsen the already hypovolaemic state of DKA, particularly where there is nausea and decreased oral intake. Hypovolaemia activates the adrenergic nervous system, further increasing insulin resistance, lipolysis and ketogenesis [19]. SGLT2 inhibitors may also decrease urinary excretion of ketones, thus increasing ketone levels in the blood [25]. Partly because of these safety concerns, SGLT2 inhibitors are not currently approved by regulatory authorities for use in type 1 diabetes.
Amputation
In the CANVAS Program, participants treated with canagliflozin were at higher risk of lower-extremity amputation than those receiving placebo (6.3 vs 3.4 participants affected per 1000 person-years; HR 1.97 [95% CI 1.41, 2.75]), with 71% of the affected participants having their highest amputation at the level of the toe or metatarsal [13]. The highest absolute risk of amputation occurred among individuals who had a history of amputation or peripheral vascular disease (PVD) but the relative risk of amputation with canagliflozin vs placebo was similar across these subgroups. The reason for the increased risk of amputation with this agent remains unclear. Additional analyses are needed to understand the potential mechanism underlying amputations with canagliflozin.
This adverse effect has not been reported with other SGLT2 inhibitors. A post hoc analysis of the EMPA-REG OUTCOME data showed that lower-limb amputation occurred in 1.9% of participants treated with empagliflozin and 1.8% of individuals treated with placebo, with an incidence rate of 6.5 per 1000 person-years in both groups [26]. When time to first event was analysed, the risk of lower-limb amputation was similar between the empagliflozin pooled group and placebo (HR 1.00 [95% CI 0.70, 1.44]). The finding was consistent across subgroups by established risk factors for amputation. Data pooled from randomised controlled trials of dapagliflozin vs placebo or vs another glucose-lowering agent showed that the risk of lower-limb amputation was similar after dapagliflozin treatment (0.1%) and control treatment (0.2%) [27].
Skeletal fractures
Canagliflozin may also increase the risk of bone fractures. In the CANVAS Program the rate of all fractures was higher with canagliflozin vs placebo (15.4 vs 11.9 participants with fracture per 1000 person-years; HR 1.26 [95% CI 1.04, 1.52]). Findings were similar with respect to low-trauma fracture events (11.6 vs 9.2 participants with fracture per 1000 person-years; HR 1.23 [95% CI 0.99, 1.52]). There was, however, evidence of heterogeneity in the findings between the two trials comprising the CANVAS Program (CANVAS and CANVAS-R), with both low-trauma fractures and all fractures occurring at greater frequency in the canagliflozin vs placebo groups in CANVAS but not in CANVAS-R [13]. This is surprising since in CANVAS-R participants might be considered at higher fracture risk owing to their greater degree of renal function impairment. The increase in fracture risk with canagliflozin was observed within the first few weeks of initiation, with a continued rate of increase thereafter [28].
An analysis of data on canagliflozin use from nine randomised controlled studies, including CANVAS, showed a higher incidence of fractures with canagliflozin (2.7%) vs comparators (1.9%) in the overall population, but this was driven by the CANVAS data [28].
The cause of the increased fracture risk with canagliflozin is unknown. Canagliflozin is not likely to have a direct effect on the skeleton via SGLT2 as the transporter is not found in bone. A possible explanation, particularly for fractures occurring after only a few months of drug exposure, is orthostatic hypotension resulting in postural dizziness and falls (CANVAS found an increased risk of volume depletion in the canagliflozin-treated group, see below for more information). Additionally, canagliflozin could potentially negatively affect bone density and bone metabolism. A randomised study showed that administration of canagliflozin doses of 100 and 300 mg were associated with a decrease in total hip bone mineral density over 104 weeks, (placebo-subtracted changes: −0.9% and −1.2%, respectively); bone mineral density was not affected at other sites measured. No meaningful changes in bone strength assessed by quantitative computed tomography finite element analysis were observed. Canagliflozin was associated with an increase in bone resorption (as suggested by an increase in collagen type 1 β-carboxy-telopeptide), an increase in bone formation (as suggested by an increase in osteocalcin) and, in women, a decrease in oestradiol levels [29]. An indirect effect of canagliflozin on bone through alterations in calcium homeostasis is also unlikely as in phase 2 clinical studies in individuals with type 2 diabetes and overweight/obese non-diabetic individuals canagliflozin treatment was not associated with meaningful changes in serum calcium, phosphorus, parathyroid hormone or 25- or 1,25-dihydroxyvitamin D [30, 31].
In the EMPA-REG OUTCOME study the proportion of participants who developed fractures was low in both the pooled empagliflozin group and the placebo group (3.8% and 3.9%, respectively) [8].
A meta-analysis of trials evaluating combined safety outcomes of canagliflozin, dapagliflozin and empagliflozin did not support a harmful effect of SGLT2 inhibitors on bone [32]. The fracture event rate was 1.59% in the SGLT2 inhibitor group and 1.56% in the control group. Moreover, the incidence of fracture events was similar among the three SGLT2 inhibitors.
Volume depletion
SGLT2 inhibitors lower BP by inducing osmotic diuresis. This effect is beneficial in individuals with uncontrolled hypertension but can lead to postural dizziness, orthostatic hypotension and dehydration, especially in elderly individuals with kidney disease or those taking loop diuretics.
In the EMPA-REG OUTCOME trial, adverse events consistent with volume depletion were rare and occurred at similar frequency in the empagliflozin and placebo groups (5.1% vs 4.9%, respectively) [8]. In contrast, in CANVAS, volume depletion was more common in the canagliflozin vs placebo group (26 vs 18.5 events per 1000 person-years; p = 0.009) [13].
Acute kidney injury
Reports suggesting a possible association between SGLT2 inhibitors and acute renal failure led the US Food and Drug Administration to issue an alert regarding the increased risk of acute kidney injury (AKI) with canagliflozin and dapagliflozin. Most reported incidences occurred within 1 month of starting therapy and improved following discontinuation, although some individuals required hospitalisation and dialysis. Volume depletion, hypotension or concomitant use of other nephrotoxic medications could have contributed to the kidney injury. An analysis of SGLT2 users and non-users in two different cohorts showed that the risk of AKI was not increased with SGLT2 inhibitor therapy [33]. Indeed, some studies support the beneficial effect of empagliflozin and canagliflozin on the progression of nephropathy and a renal protective effect after an initial small reduction in eGFR [13, 17].
Risk of cancer
Bladder and breast cancer concerns were raised with dapagliflozin due to the data from early trials included in the drug application submitted to the US Food and Drug Administration in 2011. Subsequent data submitted in 2013, which included additional trials, showed no overall imbalance of malignancies, however [34].
A recent meta-analysis of randomised controlled trials reporting cancer events in individuals treated with SGLT2 inhibitors for at least 24 weeks showed that, compared with placebo or other glucose-lowering treatments, SGLT2 inhibitors were not significantly associated with an increased risk of overall cancer (OR 1.14 [95% CI 0.96, 1.36]), although an increased risk of bladder cancer was found (OR 3.87 [95% CI 1.48, 10.08]), especially with empagliflozin (OR 4.49 [95% CI 1.21, 16.73]) [35]. However, the EMPA-REG OUTCOME investigators commented that the authors did not include all bladder cancer events from this, the largest and longest empagliflozin trial to date [36]. Herein, bladder cancer with an onset after 6 months of cumulative exposure to study drug was reported in 0.2% of participants in both the placebo and the pooled empagliflozin groups. The EMPA-REG OUTCOME data thus did not support any association between bladder cancer and empagliflozin. It should also be noted that a detection bias may occur with pharmaceuticals that are associated with urinary symptoms. Importantly, the potential carcinogenic effects of any drug cannot be easily assessed in short-term trials or even in those of a few years duration. Interestingly, in the aforementioned meta-analysis [35], canagliflozin appeared to protect against gastrointestinal cancers (OR 0.15 [95% CI 0.04, 0.60]) but this finding is likely spurious, based on the known biology of cancer.
The place of SGLT2 inhibitors in diabetes management
In a 2015 update to the 2012 ADA–EASD position statement on the management of hyperglycaemia in individuals with type 2 diabetes, SGLT2 inhibitors are suggested as second- or third-line agents after metformin in individuals needing additional glucose control [37]. In the 2018 ADA diabetes care guidelines they are recommended as second-line agents after metformin, as they are proven to reduce MACE and/or cardiovascular mortality in individuals with type 2 diabetes and established CVD (Fig. 2) [38]. Based on available data, two members of the SGLT2 inhibitor class, empagliflozin and canagliflozin are now proven to have an overall cardiovascular benefit (with only empagliflozin reducing mortality), while cardiovascular protection with other SGLT2 inhibitors is not yet established. The proposed benefits of these drugs on renal outcomes has not yet been acknowledged in treatment algorithms, to our knowledge.
Summary of latest ADA guidelines for the use of glucose-lowering drugs in individuals with type 2 diabetes in monotherapy and dual combination therapy. These are an adaptation of the most recent ADA–EASD position statement on this topic from 2015, now taking new cardiovascular data into account [38]. If HbA1c is not adequately controlled by lifestyle interventions and metformin, add a second agent. The decision on which drug to add should be based on the presence or absence of known atherosclerotic CVD. If present, choose an agent that has been demonstrated to reduce MACE and/or cardiovascular mortality in a large, randomised outcomes trial. At the time of writing this manuscript, these drugs include the SGLT2 inhibitors empagliflozin (MACE and cardiovascular mortality) and canagliflozin (MACE) and the glucagon-like peptide-1 receptor agonists liraglutide (MACE and cardiovascular mortality) and semaglutide (MACE). Guidelines currently do not incorporate other secondary outcomes from these trials, such as reductions in heart failure hospitalisation and the progression of chronic kidney disease (both of which favour the SGLT2 inhibitors). If atherosclerotic CVD is not present, any of six drug classes may be used, with the choice based on a variety of individual factors and drug-specific effects, including potency, non-glycaemic benefits, prevailing contraindications, likelihood and potential impact of adverse events and cost. ASCVD, atherosclerotic CVD; DPP-4i, DPP-4 inhibitor; GLP1-RA, glucagon-like peptide-1 receptor agonist; SGLT2i, SGLT2 inhibitor; SU, sulfonylurea; TZD, thiazolidinedione. This figure is available as part of a downloadable slideset
In addition to considering expert recommendations and national guidelines, prescribers and their patients should carefully weigh the now-recognised benefits of SGLT2 inhibitors against their safety concerns. Based on available evidence, it is reasonable to consider empagliflozin or canagliflozin as a second-line treatment for type 2 diabetes in individuals unable to achieve optimal glycaemic control with metformin, especially in those who may benefit from weight loss and/or BP reduction. These drugs appear to be particularly advantageous in those with established CVD and/or underlying prevalent kidney disease, so long as the eGFR is adequate. Despite the EMPA-REG OUTCOME trial demonstrating that mortality and chronic kidney disease benefits were observed down to an eGFR of 30 ml min−1 [1.73 m]−2, current labelling states that empagliflozin and canagliflozin should be discontinued when the eGFR is <45 ml min−1 [1.73 m]−2 and that dapagliflozin should be stopped when the GFR is <60 ml min−1 [1.73 m]−2. Use below these cut-points would be considered experimental until more information is garnered from ongoing trials. Further, it is not yet known whether these drugs might provide similar benefits if used in individuals not requiring additional glucose lowering on their current therapy.
The post hoc analyses from CANVAS suggesting a heart failure and renal benefit even in those without prevalent CVD should also be considered hypothesis-generating at this point and need to be substantiated by additional studies. It is difficult to know whether other SGLT2 inhibitors, namely dapagliflozin and ertugliflozin, will have the same benefits, as cardiovascular outcome trials involving these drugs are still pending. Their risk profile also needs further elucidation.
Canagliflozin should be avoided in individuals at risk for amputations, such as those with advanced PVD, severe peripheral neuropathy or prior history of lower-limb amputation or foot ulceration. Even though amputations were not seen in the EMPA-REG OUTCOME trial, it is not unreasonable to avoid the entire class in those at the highest amputation risk until more safety data are accumulated. Canagliflozin should probably also be used cautiously in those with prior fragility fractures or at high fracture risk, such as women with osteoporosis.
Although the trend in stroke in the EMPA-REG OUTCOME trial might suggest the drug be avoided in those with cerebrovascular disease, further analysis revealed that the imbalance in stroke events was largely due to occurrences in participants already off the study drug for at least 90 days. Accordingly, it is difficult to conclude that the drug specifically increases stroke risk. In addition, the trend for cardiovascular mortality was similar in individuals with and without a history of stroke at baseline [8].
SGLT2 inhibitors are to be avoided in individuals with prior history of complicated UTIs such as pyelonephritis or urosepsis, those with an indwelling urinary catheter and those with recurrent genital mycotic infections. Men with prostatic hypertrophy and women with urinary incontinence will find this drug class challenging to use, and this is likely to have a negative impact on quality of life. Caution should be taken before initiating SGLT2 inhibitor therapy in individuals with a predisposition to hypovolaemia, those who have borderline kidney function or those taking concomitant potent diuretics or nephrotoxic medications such as non-steroidal anti-inflammatory drugs.
The diuretic effect exerted by SGLT2 inhibitors means it is important to assess volume status and BP before initiating treatment. In hypovolaemic or hypotensive individuals, the SGLT2 inhibitor should be delayed until the fluid status and BP are corrected. If an individual is euvolaemic but verging towards hypotension, SGLT2 inhibitors should be initiated cautiously and close monitoring is advised. Euvolaemic, normotensive individuals on thiazide therapy can continue with the thiazide as this diuretic does not further reduce BP beyond the reduction achieved with SGLT2 inhibitors. In contrast, in individuals receiving more potent loop diuretics, reducing the dose by 50% should be considered, with close follow-up. In individuals with heart failure, any such changes would ideally be made after consultation with a cardiologist. Individuals on concurrent SGLT2 inhibitor and any diuretic should have their BP, weight, creatinine and electrolytes measured periodically. Finally, SGLT2 inhibitors should not be started in individuals with labile volume status [39].
Despite possible glycaemic benefits, SGLT2 inhibitors should not be used in type 1 diabetes because of concerns regarding euglycaemic DKA; individuals with latent autoimmune diabetes of adults (LADA) may also be at higher risk of developing ketoacidosis. Avoidance of SGLT2 inhibitors is advisable in individuals with type 2 diabetes who have evidence of very low insulin secretory capacity, as suggested by labile diabetes control, lean body build and, of course, prior episodes of ketosis.
Finally, since SGLT2 inhibitors are relatively new and are thus far only available as proprietary brand formulations, cost should also be considered. In the USA, for example, retail costs are more than 100-fold higher than for generic metformin or sulfonylureas. Justifying such expenses, using the information from clinical trials available to date, may be difficult for the more typical newly diagnosed individuals with type 2 diabetes who do not have prevalent CVD or nephropathy. Benefit in this group will need to be proven.
Conclusions
SGLT2 inhibitors, constituting the most recently available oral glucose-lowering drug category, exert their effect by increasing urinary glucose excretion. Optimal prescribing of agents within this category requires a full understanding of their risks in addition to their benefits. Besides improving glycaemic control, weight and BP, some members of this class provide beneficial cardiovascular and renoprotective effects. SGLT2 inhibitors should be considered reasonable second-line treatment options for individuals at risk of cardiovascular events or those with underlying nephropathy, if good glycaemic control has not been achieved with metformin monotherapy. Possible side effects include urinary frequency and orthostasis. Additional potential adverse effects include genitourinary tract infections, euglycaemic DKA, AKI, lower-extremity amputations and fractures—the latter two to date being associated solely with canagliflozin. Individuals at risk for such complications should be monitored closely and treatment should be discontinued or at least reconsidered if they occur.
Abbreviations
- AKI:
-
Acute kidney injury
- CANVAS:
-
Canagliflozin Cardiovascular Assessment Study
- CANVAS-R:
-
CANVAS-Renal
- CVD:
-
Cardiovascular disease
- DKA:
-
Diabetic ketoacidosis
- DPP-4:
-
Dipeptidyl peptidase-4
- EMPA-REG OUTCOME:
-
Empagliflozin, Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients
- MACE:
-
Major adverse cardiac events
- PVD:
-
Peripheral vascular disease
- SGLT2:
-
Sodium–glucose cotransporter 2
- UTI:
-
Urinary-tract infection
References
Stenlof K, Cefalu WT, Kim KA et al (2013) Efficacy and safety of canagliflozin monotherapy in subjects with type 2 diabetes mellitus inadequately controlled with diet and exercise. Diabetes Obes Metab 15:372–382
Roden M, Weng J, Eilbracht J et al (2013) Empagliflozin monotherapy with sitagliptin as an active comparator in patients with type 2 diabetes: a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Diabetes Endocrinol 1:208–219
Ferrannini E, Ramos SJ, Salsali A, Tang W, List JF (2010) Dapagliflozin monotherapy in type 2 diabetic patients with inadequate glycemic control by diet and exercise: a randomized, double-blind, placebo-controlled, phase 3 trial. Diabetes Care 33:2217–2224
Terra SG, Focht K, Davies M et al (2017) Phase III, efficacy and safety study of ertugliflozin monotherapy in people with type 2 diabetes mellitus inadequately controlled with diet and exercise alone. Diabetes Obes Metab 19:721–728
Wang Z, Sun J, Han R et al (2018) Efficacy and safety of sodium-glucose cotransporter-2 inhibitors versus dipeptidyl peptidase-4 inhibitors as monotherapy or add-on to metformin in patients with type 2 diabetes mellitus: a systematic review and meta-analysis. Diabetes Obes Metab 20:113–120
Ridderstrale M, Andersen KR, Zeller C, Kim G, Woerle HJ, Broedl UC (2014) Comparison of empagliflozin and glimepiride as add-on to metformin in patients with type 2 diabetes: a 104-week randomised, active-controlled, double-blind, phase 3 trial. Lancet Diabetes Endocrinol 2:691–700
Pinto LR, Rados DV, Remonti LR, Kramer CK, Leitao CB, Gross JL (2015) Efficacy of SGLT2 inhibitors in glycemic control, weight loss and blood pressure reduction: a systematic review and meta-analysis. Diabetol Metab Syndr 7(Suppl 1):A58
Zinman B, Wanner C, Lachin JM et al (2015) Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 373:2117–2128 including supplemental appendix
Inzucchi SE, Zinman B, Fitchett D et al (2018) How does empagliflozin reduce cardiovascular mortality? Insights from a mediation analysis of the EMPA-REG OUTCOME trial. Diabetes Care 41:356–363
Ferrannini E, Mark M, Mayoux E (2016) CV protection in the EMPA-REG OUTCOME trial: a ‘thrifty substrate’ hypothesis. Diabetes Care 39:1108–1114
Baartscheer A, Schumacher CA, Wust RC et al (2017) Empagliflozin decreases myocardial cytoplasmic Na+ through inhibition of the cardiac Na+/H+ exchanger in rats and rabbits. Diabetologia 60:568–573
Zinman B, Inzucchi SE, Lachin JM et al (2017) Empagliflozin and cerebrovascular events in patients with type 2 diabetes mellitus at high cardiovascular risk. Stroke 48:1218–1225
Neal B, Perkovic V, Mahaffey KW et al (2017) Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 377:644–657
Mahaffey KW, Neal B, Perkovic V et al (2018) Canagliflozin for primary and secondary prevention of cardiovascular events: results from the CANVAS Program (canagliflozin cardiovascular assessment study). Circulation 137:323–334
Kosiborod M, Cavender MA, Fu AZ et al (2017) Lower risk of heart failure and death in patients initiated on sodium-glucose cotransporter-2 inhibitors versus other glucose-lowering drugs: the CVD-REAL study (comparative effectiveness of cardiovascular outcomes in new users of sodium-glucose cotransporter-2 inhibitors). Circulation 136:249–259
Birkeland KI, Jorgensen ME, Carstensen B et al (2017) Cardiovascular mortality and morbidity in patients with type 2 diabetes following initiation of sodium-glucose co-transporter-2 inhibitors versus other glucose-lowering drugs (CVD-REAL Nordic): a multinational observational analysis. Lancet Diabetes Endocrinol 5:709–717
Wanner C, Inzucchi SE, Lachin JM et al (2016) Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med 375:323–334
Vasilakou D, Karagiannis T, Athanasiadou E et al (2013) Sodium-glucose cotransporter 2 inhibitors for type 2 diabetes: a systematic review and meta-analysis. Ann Intern Med 159:262–274
Peters AL, Buschur EO, Buse JB, Cohan P, Diner JC, Hirsch IB (2015) Euglycemic diabetic ketoacidosis: a potential complication of treatment with sodium-glucose cotransporter 2 inhibition. Diabetes Care 38:1687–1693
Palmer BF, Clegg DJ, Taylor SI, Weir MR (2016) Diabetic ketoacidosis, sodium glucose transporter-2 inhibitors and the kidney. J Diabet Complicat 30:1162–1166
Fralick M, Schneeweiss S, Patorno E (2017) Risk of diabetic ketoacidosis after initiation of an SGLT2 inhibitor. N Engl J Med 376:2300–2302
Ferrannini E, Muscelli E, Frascerra S et al (2014) Metabolic response to sodium-glucose cotransporter 2 inhibition in type 2 diabetic patients. J Clin Invest 124:499–508
Merovci A, Solis-Herrera C, Daniele G et al (2014) Dapagliflozin improves muscle insulin sensitivity but enhances endogenous glucose production. J Clin Invest 124:509–514
Keller U, Schnell H, Sonnenberg GE, Gerber PP, Stauffacher W (1983) Role of glucagon in enhancing ketone body production in ketotic diabetic man. Diabetes 32:387–391
Taylor SI, Blau JE, Rother KI (2015) SGLT2 Inhibitors may predispose to ketoacidosis. J Clin Endocrinol Metab 100:2849–2852
Inzucchi SE, Iliev H, Pfarr E, Zinman B (2018) Empagliflozin and assessment of lower-limb amputations in the EMPA-REG OUTCOME trial. Diabetes Care 41:e4–e5
Jabbour S, Seufert J, Scheen A, Bailey CJ, Karup C, Langkilde AM (2018) Dapagliflozin in patients with type 2 diabetes mellitus: a pooled analysis of safety data from phase IIb/III clinical trials. Diabetes Obes Metab 20:620–628
Watts NB, Bilezikian JP, Usiskin K et al (2016) Effects of canagliflozin on fracture risk in patients with type 2 diabetes mellitus. J Clin Endocrinol Metab 101:157–166
Bilezikian JP, Watts NB, Usiskin K et al (2016) Evaluation of bone mineral density and bone biomarkers in patients with type 2 diabetes treated with canagliflozin. J Clin Endocrinol Metab 101:44–51
Rosenstock J, Aggarwal N, Polidori D et al (2012) Dose-ranging effects of canagliflozin, a sodium-glucose cotransporter 2 inhibitor, as add-on to metformin in subjects with type 2 diabetes. Diabetes Care 35:1232–1238
Bays HE, Weinstein R, Law G, Canovatchel W (2014) Canagliflozin: effects in overweight and obese subjects without diabetes mellitus. Obesity 22:1042–1049
Tang HL, Li DD, Zhang JJ et al (2016) Lack of evidence for a harmful effect of sodium-glucose co-transporter 2 (SGLT2) inhibitors on fracture risk among type 2 diabetes patients: a network and cumulative meta-analysis of randomized controlled trials. Diabetes Obes Metab 18:1199–1206
Nadkarni GN, Ferrandino R, Chang A et al (2017) Acute kidney injury in patients on SGLT2 inhibitors: a propensity-matched analysis. Diabetes Care 40:1479–1485
Center for Drug Evaluation and Research (2013) Application number: 2022930Org1s000. Available from https://www.accessdata.fda.gov/drugsatfda_docs/nda/2014/202293Orig1s000SumR.pdf
Tang H, Dai Q, Shi W, Zhai S, Song Y, Han J (2017) SGLT2 inhibitors and risk of cancer in type 2 diabetes: a systematic review and meta-analysis of randomised controlled trials. Diabetologia 60:1862–1872
Kohler S, Lee J, George JT, Inzucchi SE, Zinman B (2017) Bladder cancer in the EMPA-REG OUTCOME trial. Diabetologia 60:2534–2535
Inzucchi SE, Bergenstal RM, Buse JB et al (2015) Management of hyperglycaemia in type 2 diabetes, 2015: a patient-centred approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetologia 58:429–442
Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes-2018. Diabetes Care 41: S73-S85
Cherney DZ, Udell JA (2016) Use of sodium glucose cotransporter 2 inhibitors in the hands of cardiologists: with great power comes great responsibility. Circulation 134:1915–1917
Author information
Authors and Affiliations
Contributions
Both authors were responsible for drafting and revising the article and approved this version for publishing.
Corresponding author
Ethics declarations
SI has consulted for Janssen and vTv Pharmaceuticals and served on Research Steering Committees for AstraZeneca, Boehringer Ingelheim, Daiichi Sankyo, Novo Nordisk and Sanofi/Lexicon, Eisai (TIMI) and on a Data Monitoring Committee for Intarcia. BL declares that there is no duality of interest associated with her contribution to this manuscript.
Electronic supplementary material
Slideset of figures
(PPTX 187 kb)
Rights and permissions
About this article
Cite this article
Lupsa, B.C., Inzucchi, S.E. Use of SGLT2 inhibitors in type 2 diabetes: weighing the risks and benefits. Diabetologia 61, 2118–2125 (2018). https://doi.org/10.1007/s00125-018-4663-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-018-4663-6