Abstract
Monitoring levels of biologicals against tumor necrosis factor (TNF) has been suggested to improve therapeutic outcomes in inflammatory bowel diseases (IBDs). This pilot study describes a rapid lateral flow (LF)-based assay for on-site monitoring of serum trough levels of humanized monoclonal antibody infliximab (IFX). The applied chromatographic method utilizes sequential flows of diluted serum, wash buffer, and an immunoglobulin generic label on LF strips with a Test line comprised of TNF-α. The successive flows permitted enrichment of IFX at the Test line before the label was applied. The label, luminescent upconverting phosphor (UCP) particles coated with protein-A, emits a 550-nm visible light upon excitation with 980-nm infrared light. IFX concentrations were determined through measurement of UCP fluorescence at the Test line. The assay was optimized to detect IFX levels as low as 0.17 μg/mL in serum. For patients with IBD, this limit is appropriate to detect levels associated with loss of response (0.5 μg IFX/mL). The assay was evaluated with clinical samples from patients with Crohn’s disease and correlated well within the physiologically relevant range from 0.17 to 10 μg/mL with an IFX-specific ELISA. Performance of the assay was further successfully validated with samples from blood donors, IFX negative IBD patients, and rheumatoid arthritis patients that had developed anti-IFX antibodies. Because of its generic nature, the assay is suited for detecting most therapeutic anti-TNF-α monoclonal antibodies.

A rapid lateral flow based assay to determine trough levels of infliximab and other anti‐TNF‐α antibodies. The rapid format showed excellent and quantitative correlation with ELISA. Accurate quantitation was achieved utilizing the up‐converting phosphor reporter technology and a portable lightweight ESEQuant LFR reader adapted with an infrared LED
Similar content being viewed by others
Introduction
Therapeutic monoclonal antibodies such as infliximab (Remicade®) and adalimumab (Humira®) have profoundly changed the treatment of autoimmune diseases such as inflammatory bowel disease (IBD) and rheumatoid arthritis (RA) [1–3]. In patients with moderate-to-severe Crohn’s disease (CD) and ulcerative colitis (UC), infliximab (IFX) treatment is effective both as induction and as maintenance therapy in patients that do not respond to conventional therapy [1]. In rheumatoid arthritis, anti-tumor necrosis factors (TNFs) have been shown to rapidly improve symptoms, retard radiographic disease progression, and to improve functional status and health-related quality of life [4].
Unfortunately, a substantial proportion of patients that initially respond to IFX lose response as a result of antibody formation to IFX and, consequently, enter a state of inadequate therapeutic levels of the drug [4–7]. Antibodies may be detected in up to 61 % of episodically treated IBD patients [4] and in 16 % of patients on scheduled maintenance therapy [8]. Formation of antibodies and undetectable levels of IFX are associated with allergic reactions, loss of response, and unfavorable clinical outcome [4, 5, 9–13]. Therapeutic monitoring of IFX has been advocated to optimize the use of anti-TNFs [14]. Monitoring the IFX levels allows determination of the lowest IFX dose where it is still able to exert its full effects though avoiding overdosing that may be associated with increased toxicity. This will ultimately lead to a more cost-effective utilization of the drug and is becoming a part of routine clinical management [15, 16].
Testing of IFX levels is mostly performed using blood samples collected immediately before administration of a new infusion, so-called trough levels. Current testing requires a dedicated ELISA and is only performed by specialized laboratories. As a consequence, test results are not immediately available to act upon when administrating the scheduled infusion. Tools that allow rapid and more frequent monitoring would permit a better and personalized administration of the drugs based on actual trough levels.
Here, we describe the application of a rapid, lateral flow-based assay format for detection and quantification of IFX trough levels in serum. IFX present is captured at the Test line of the LF strip comprised of TNF-α, and subsequently detected using the high sensitivity upconverting phosphor (UCP) reporter technology [17]. UCP reporter particles are composed of rare earth lanthanide elements embedded in a crystal. They exhibit anti-Stokes behavior, low energy infrared light (980 nm) is upconverted to visible light of higher energy; the particles used in this study emit 550 nm green light. Upconversion provides an unmatched contrast as the process is restricted to the crystal lattice and autofluorescence of other assay components thus is entirely absent. The combination of the UCP reporter technology with the user-friendly and rapid LF-based immunochromatography has shown better sensitivity than ELISA-based assays, and several UCP-LF applications to detect different types of biomolecules have already been described (references recapped in Corstjens et al. 2011 [18]). In the current study, the focus is on IFX and IBD, but the results indicate that the assay in its current form is directly applicable to other biologicals (immunotherapeutics) and diseases as e.g. adalimumab (ADA) and RA.
Methods
Patient population
A series of 84 clinical samples from patients with Crohn’s disease was used to compare the performance of newly developed UCP lateral flow test with an IFX-specific ELISA. Serum was obtained from patients on scheduled maintenance IFX therapy for Crohn’s disease in the Leiden University Medical Center. The study was approved by the Commission for Medical Ethics of LUMC and performed accordingly. After obtaining informed consent, blood was drawn immediately before infliximab infusion at two consecutive visits. Blood was collected in standard vacutainers for serum; serum was centrifuged and stored in aliquots at −20 °C. Other series used for further validation of the assay included banked serum samples of 12 IBD patients (previously not treated with any biological, LUMC Gastroenterology and Hepatology), 95 healthy blood bank donors (LUMC Parasitology) as well as 29 RA patients who (previously) received IFX with various levels of ATI (Sanquin).
Materials and reagents
Protein-A (recombinant, #RPA-50) obtained from Repligen Corp., Waltham, MA, USA, was covalently bound to the UCP reporter (300 to 400 nm Y2O2S:Yb3+,Er3+ particles; OraSure Technologies Inc., Bethlehem, PA) in a ratio of 25 μg protein per mg particles as previously described [19]. The silicated particles with C10-carboxylated functional surface groups were coated with protein-A utilizing a NHS (sulfo-N-hydroxysuccinimide) activated and EDC (1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride) mediated reaction allowing the formation of amide linkages from a reaction of the carboxylate groups on the surface of the UCP reporter and (primary) amine groups of the protein. Recombinant (yeast), research grade human TNF-α (#130-094-018) was obtained from Miltenyi Biotec GmbH, Bergisch Gladbach, Germany. Polyclonal rabbit anti-goat antibodies (Sigma #G4018) and Albumin Bovine Fraction V (BSA, Sigma #A2153) were purchased from Sigma-Aldrich Co. LLC. Pooled normal human serum (NHS) from Innovative Research was obtained through Dunn Labortechnik GmbH, Asbach, Germany. Infliximab (IFX, Remicade®) was obtained from Centocor BV, Leiden, the Netherlands.
Lateral flow strips for quantification of IFX
LF strips were assembled as described earlier [19, 20]; 4 mm width and 5 cm in length, consisting of 1 cm sample pad, 2.5 cm nitrocellulose, and 2 cm absorbent pad. Sample and absorbent pad each overlap 2.5 mm with the nitrocellulose membrane. The nitrocellulose was provided with a Test line comprised of TNF-α (50 to 400 ng per 4 mm width) at 15 mm and a Flow Control line at 20 mm comprised of 100 ng rabbit anti-goat antibody.
UCP-CF assay for IFX
The UCP assay consists of three sequential flow steps (consecutive flow, CF, Fig. 1). In the first step, 40 μL “sample” is added to the LF strip. The “sample” either is 40 μL high salt lateral flow assay buffer (HSLF, 100 mM Hepes pH 7.5, 270 mM NaCl, 0.5 % (v/v) Tween-20, 1 % (w/v) BSA) spiked with IFX or a fivefold dilution of serum (8 μL) in HSLF (32 μL). The second step is a wash step with 20 μL HSLF, initiated immediately after the 40-μL “sample” has been absorbed by the sample pad. The wash removes excess of antibodies to minimize potential interaction of the protein A UCP conjugate with unbound antibodies. The first flow step (“sample”) takes about 1 min, the second flow step (wash) is allowed to continue for 4 min. In the third flow step, 70 μL of reporter (100 ng of the protein A UCP conjugate in HSLF) is applied to the strip, and immunochromatography is allowed to continue for at least 15 min. LF strips can be scanned after a total assay time of 20 min. Longer incubations allowing complete drying of the LF strips will result in higher signals measured at Test (T) and Flow Control (FC) lines, but it does not change the ratio value that is calculated by dividing the T signal by the FC signal. Signals are measured as relative fluorescent units (RFUs) representing the intensity of the emitted green light upon excitation of the UCP reporter particles captured in the T and FC zone. Assay results are generally presented as ratio values as this increased dynamic range and improved assay precision [6]. Ratio values determined for single blind tested clinical samples, consequently with unknow IFX values, were correlated to IFX concentration values determined at Sanquin with an IFX-specifc ELISA. Scanning of the LF strips was performed with an adapted Packard FluoroCountTM microtiter plate reader provided with a 980-nm infrared laser [17, 21] and a custom designed lightweight (handheld) portable UCP-Quant reader (Qiagen Lake Constance GmbH, Stockach, Germany). The UCP-Quant reader can be used as a standalone device, thus well suited for on-site testing. Like the earlier described UPlink reader [22], the UCP Quant reader is developed for the analysis of a single strip.
Schematic illustration of the IFX UCP-CF assay. The CF format comprises three sequential flow steps. The lateral flow steps are performed by placing LF strips upright in microtiterplate wells with sequential addition of the appropriate solutions to the microtiterplate well. Upon completion of the third flow step, strips are scanned with readers containing an IR light excitation source required for the UCP reporter technology. Antibodies representing IFX are indicated with solid lines, antibodies representing all other antibodies (human Igs) present in a serum sample are indicate with dashed lines
IFX dilution series for analytical sensitivity and comparison with ELISA
Analytical sensitivity
The analytical sensitivity of the UCP-CF assay was determined with IFX dilution series in HSLF. The assay allowed addition of 40 μL spiked HSLF per LF strip in the first flow step (sample flow). When using clinical samples, a dilution factor needs to be included. The standard protocol required addition of a fivefold diluted sample which is equal to an input of 8 μL serum. Sample dilution factors are dependent on the type of the biological matrix; the assay format is also applicable to plasma, saliva, and urine.
Comparison of UCP-CF and ELISA
Quality control standards (dilution series) of IFX in NHS were prepared at LUMC to compare the performance of the IFX ELISA and UCP-CF assay. ELISA assays were performed at Sanquin Research (Amsterdam, Netherlands) on serum samples according to standard protocol [23] testing twofold serial dilutions in high-performance ELISA buffer (HPE, Business Unit Reagents, Sanquin). Clinical samples were tested with the same protocol as the quality control standards; IFX concentrations were determined by fitting OD values to results obtained with the IFX standard dilution series using four or five parameter curve fitting. ATI were measured at Sanquin Research according to published methods [24].
Results
The UCP consecutive flow (UCP-CF) assay format
The specificity of UCP-CF assays detecting human antibodies were determined solely by the capture molecule immobilized on the Test line. Previously, the UCP-CF format was applied for the detection of human IgG antibodies against HIV, HCV, and TB antigens [25] and IgM antibodies against Mycobacterium leprae glycolipid PGL-I [18]. Here, we describe a new application of the UCP-CF assay format, determination and quantification of trough levels of the immunotherapeutic IFX. The developed IFX UCP-CF assay was validated by comparison to ELISA utilizing a set of clinical samples from Crohn’s disease patients. A benchtop protocol applicable for testing multiple samples was applied. LF strips were scanned in a multi-strip reader and a lightweight portable reader dedicated for single strip scanning. Results obtained with both readers correlated well; a coefficient of determination (R 2) value >0.97 between the two readers was determined. Minor differences were explained by differences in software protocols used to calculate peak area values.
Analytical sensitivity
The analytical sensitivity of the UCP-CF IFX assay was determined in a clean system (buffer only) without restrictions related to the matrix of the clinical sample. This implied detection of IFX spiked in a 40-μL assay buffer; 40 μL being the maximum volume of the first flow (sample flow, Fig. 1) and thus theoretical maximum amount of “sample” that can be added in the assay. Besides sample size, the sensitivity of the assay was dependent on, and could be controlled by, the amount of TNF-α immobilized on the Test (T) line. Other factors are the distance of T from the sample pad and the amount of UCP label used in the assay. Standard conditions, as determined with other UCP-CF antibody detection assays, implied the use of 100 ng UCP-conjugate and LF strips with a T line comprised of 100 ng capture protein. These conditions were used to investigate feasibility of the assay by determining the detection limit of IFX in buffer. A serial dilution (increments of 3) of IFX was tested in triplicate and allowed detection down to 0.008 μg/mL IFX (Fig. 2). The average ratio value obtained with the lowest amount of IFX (0.008 μg/mL) tested was 0.029 compared to 0.010 for the zero control sample without IFX. These results indicated good feasibility of the assay to discriminate the low- (or non-) responders from intermediate and high responders [23].
Assay sensitivity and dynamic range UCP-CF with IFX spiked in assay buffer. The analytical sensitivity of the UCP-CF IFX assay as determined without influence of biological matrix. Assay results are presented as ratio values (a) calculated by dividing the signals measured at the T and FC line (b). Signals are measured as RFUs. Error bars indicate the variation (±1 standard deviation) in the calculated ratio value for an experiment performed in triplicate
Similar experiments were repeated several times with various batches of reagents. Observed differences in the analytical sensitivity were mostly related to variation between the different lots of manually produced LF strip. In order to maintain the same assay sensitivity, the amount of TNF-α immobilized on the Test lined was varied between 50 and 400 ng (per 4 mm strip width). Results obtained with different lots may also vary due to some variation in the location of T and FC lines. The quality control for different lots of strips required detection of 0.008 μg/mL IFX (in buffer) with ratio values at least two times the ratio value obtained with the zero control (buffer only).
Sample size (serum load)
The CF assay format was previously developed for detection of human antibodies against HIV and other pathogens. Serum amounts of only 0.1 to 1 μL worked well in qualitative analysis to demonstrate HIV infection. The optimal amount of or serum for a quantitative analysis of IFX levels was investigated and showed that relevantly larger amounts of serum resulted in better detection limits. Figure 3 shows the result of a test performed in duplicate using two production lots of LF strips. A dilution series of IFX spiked in NHS was tested utilizing 0.5 through 16 μL of the biological matrix (spiked NHS) in the first step of the CF (sample flow, Fig. 1); assay buffer was added to a final volume of 40 μL. The concentration of NHS in the 40-μL sample flow thus ranged of 1.25 to 40 % (v/v), whereas the concentration of IFX in NHS ranged from 40.5 to 0.17 μg/mL (serial dilution in increments of 3). The cutoff for IFX trough levels in Crohn’s disease that discriminate most accurately between response and loss of response is 0.5 μg/mL [26]. This level is detectable when the UCP-CF assay is performed with 2 μL (or more) spiked NHS. The highest ratio values were obtained with the samples containing up to 40 % of NHS, but the variation in test results increased, probably as a consequence of excess of total Ig leading to saturation of one of the assay components. Comparable experiments performed with some clinical samples showed a similar profile (results not shown).
Serum sample size in relation to IFX detection limit. Effect of the amount of NHS in the first flow step of the UCP-CF assay. IFX was spiked in NHS at a concentration of 0 through 40.5 μg/mL. The amount serum was varied from 1.25 % through 40 % (v/v). The experiment was performed in duplicate; error bars indicate 1 standard deviation. Black bars indicate the 0.5 μg/mL threshold
Potential variability of the UCP-CF assay when applied to different individual serum samples was tested on 15 samples from healthy controls. Samples were spiked with 0.5 and 1.5 μg IFX per mL and 8 μL of spiked serum was used to perform the UCP-CF assay; the 8 μL corresponded to a serum load of 20 % in the sample flow step (see Fig. 3). The average ratio value (n = 3) for the 0, 0.5, and 1.5 μg/mL IFX was 0.015 ± 0.019, 0.095 ± 0.031, and 0.274 ± 0.055, respectively. Lower concentrations of IFX led to relatively higher standard deviations when comparing samples from different individuals. However, in all cases the 0.5-μg/mL IFX level was easily distinguished from the overall average background signal.
Comparison of the ELISA and UCP-CF assay using spiked samples
IFX dilution series spiked in serum were analyzed by UCP-CF and ELISA in single blind experiments. ELISA results were obtained from Sanquin as the concentration of IFX per milliliter of serum. ELISA values and the ratio values determined by UCP-CF were plotted against the spiked amount of IFX (Fig. 4). All samples were tested in triplicate; for the UCP-CF assay, three different batches of LF strips were used. The ELISA results indicated excellent reproducibility. The UCP-CF assay showed some more variation which was attributed to the use of different production lots of LF strips. Results indicated that a good correlation between ELISA and UCP-CF is maintained regardless of batch differences of the LF strips. At concentrations above the 40 μg/mL level, ratio values obtained with the UCP-CF indicate the presence of a plateau.
Evaluation of UCP-CF assay with clinical samples
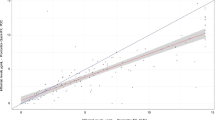
Eighty-four blood samples were analyzed in single blind experiments by ELISA (Sanquin Research) and by UCP-CF (LUMC). Calculated UCP-CF ratio values were plotted against the IFX concentrations determined by ELISA (Fig. 5a). In the physiological range of interest (between 0 and 10 μg/mL), an excellent quantitative linear correlation between the ELISA and the UCP-CF assay was found with a coefficient of determination (R 2) value of 0.85. Ranking of the samples based on their assay value in both assays (Spearman ranking, Fig. 5b) further confirmed the excellent correlation between the ELISA and UCP-CF test results.
Evaluation of UCP-CF with clinical samples. Comparison of the UCP-CF ratio values with IFX concentrations determined by ELISA. A total of 84 clinical samples were tested in a single blind experiment with the UCP-CF and ELISA. a UCP-CF ratio values plotted against the concentration determined by ELISA. b Spearman correlation by rank order based on assay value
Applicability of the 0.5-μg/mL IFX trough level loss of response was validated with serum samples from 95 healthy blood donors. For 20 samples, UCP-CF results were confirmed by ELISA. In these experiments, the UCP-CF average negative ratio value was 0.005 ± 0.007, the sample with the highest negative value was 0.029 compared to a ratio value of 0.071 ± 0.028 for NHS spiked with 0.17 μg IFX. ELISA results indicated a level below detection for all 20 samples. A separate UCP-CF test further showed that all 95 healthy blood donor samples resulted in values well below the defined loss of response IFX concentration. Similar results were obtained with a series of 12 serum samples from IBD patients without IFX drug treatment included in this test.
The performance of the UCP-CF assay with 29 samples from ATI confirmed patients (range 12–1,100, median 29, and interquartile range 20–55 arbitrary units/mL) showed an excellent correlation (R 2 = 0.98) with the ELISA when leaving out two discrepant samples. The two discrepant samples scored UCP-CF values indicating >1 μg/mL of a TNF-α-specific antibody. Further analysis with other biological-specific ELISAs revealed the presence of the biological adalimumab and etanercept in these two samples, respectively, both at levels above 2 μg/mL. This confirmed the fact that the UCP-CF assay with the Ig generic reporter (UCP coated with protein A) is applicable for detection of other anti-TNF-α biological.
Discussion
The anti-TNF antibodies are currently among the most effective drugs in the therapeutic management of IBD [1]. Recent evidence suggests that the monitoring of anti-TNF drug levels will play an emerging role in future management of IBD [27, 28]. First, correcting low levels is believed to be efficacious in maintaining adequate response to therapy [14, 15, 28, 29]. Second, identify non-response and progression of disease, whilst adequate levels of IFX indicate the necessity to switch to alternative immunosuppressive medication [14]. Third, cost-effective anti-TNF strategies are essential for controlling direct costs of IBD healthcare delivery and access to this relative expensive antibody-based therapy [15, 30]. A number of challenges will need to be overcome before optimal use of anti-TNF can be deployed universally, including practical guidelines for implementing anti-TNF drug monitoring, defining optimal cutoff levels, timing of measurement, and the necessity of concurrent measurement of antibodies against anti-TNF. In addition, the various methods that have been used in the different studies (RIA, ELISA, Western blot analysis) addressing trough level measurement often lacked standardization and proved too laborious in execution to consider applications involving rapid single-patient testing [31]. Healthcare delivery systems are changing at a very rapid pace. The need for drastic cost reduction accelerates the introduction of cost-effective care pathways supported by electronic medical records and point-of-care (POC) testing. Patient participation within these defined care pathways will become mandatory, especially in the management of chronic diseases which absorbs 70 % of healthcare budgets. Gentle steps towards homecare and self-management are currently evaluated, periodically capturing relevant clinical and laboratory parameters for monitoring disease activity and drug safety. It is with this in mind that we have evaluated a POC test for the measurement of infliximab.
In this study, we have developed and evaluated an UCP-CF IFX assay optimized to allow quantitative detection of IFX down to 0.17 μg/mL in serum samples. The assay showed a good correlation of quantitative values with a series of 84 clinical samples from CD patients when compared to an IFX-specific ELISA. In this study, the level of 0.5 μg/mL IFX was presumed for CD patients to indicate loss of response to the biological [26]; samples from a group of 95 healthy blood donors all scored well below this level. The assay could be further validated on samples from a group of ATI positive RA patients and again showed excellent correlation with ELISA as well as that it disclosed the test’s applicability to detect the presence of other TNF-α-specific biological (in this case ADA and ETA). We propose that this low-complexity UCP-CF IFX assay is an attractive and rapid alternative for current available IFX ELISAs, in particular applicable in settings that demand single sample testing. In this respect, two settings can be distinguished: (1) application of the IFX-LF test in a hospital laboratory or a physician’s office (POC) and (2) the homecare situation where patients may use the test themselves. Both situations will profit from full implementation of dry reagents, fingerstick blood sampling, and dedicated handheld readers allowing automated analysis. Several questions related to these issues have been investigated as part of other in-house assay developments, including the use of commercially available reporter technologies. For various settings, alternative assay platforms applying e.g. electrochemical [32] or magnetic-based detection [33] can be considered as well. The introduction of the test for POC laboratory applications and third party evaluation therefore is within hand’s reach; dry reagents as well as lightweight handheld readers (UCP-Quant) are available. For the homecare situation, some additional requirements are needed, as for instance the application of a web-based interface to deliver test results from home to the clinic (which was recently demonstrated) [34] or the integration of an electronic system in the reader transferring the data automatically to the hospital upon scanning of the LF strip. Moreover, further adjustments to improve robustness (e.g., the implementation of various assay controls) and ease of use of the technology are in progress.
In conclusion, in this pilot study we have evaluated the feasibility of an attractive alternative to current IFX drug level testing using an UCP-CF assay well suited for POC settings for example infusion centers. It delivers immediate access to actual trough level concentrations thereby allowing appropriate adaptation of the drug administration rather than the use of predetermined protocols. The assay proved to be able to detect other TNF-α-specific biologicals (ADA and ETA) and may have a use in monitoring trough level of biologicals in various chronic diseases. As this is a pilot study, further evaluation and integration of the test in a user-friendly device is required. Moreover, future anticipated implementation of whole blood sampling from a finger stick may allow self-test applications including monitoring by the patient at home.
Abbreviations
- ADA:
-
Adalimumab
- ATI:
-
Antibodies to infliximab
- CF:
-
Consecutive flow
- HSLF:
-
High salt lateral flow
- IBD:
-
Infectious bowl disease
- IFX:
-
Infliximab
- Ig:
-
Immunoglobulin
- LF:
-
Lateral flow
- NHS:
-
Normal human serum
- POC:
-
Point-of-care
- RA:
-
Rheumatoid arthritis
- RFU:
-
Relative fluorescent unit
- TNF-α:
-
Tumor necrosis factor alpha
- UCP:
-
Upconverting phosphor
References
Ford AC, Sandborn WJ, Khan KJ, Hanauer SB, Talley NJ, Moayyedi P (2011) Efficacy of biological therapies in inflammatory bowel disease: systematic review and meta-analysis. Am J Gastroenterol 106:644–659
Furst DE, Keystone EC, Braun J, Breedveld FC, Burmester GR, De Benedetti F et al (2010) Updated consensus statement on biological agents for the treatment of rheumatic diseases, 2010. Ann Rheum Dis 70:i2–i36
Knight DM, Trinh H, Le J, Siegel S, Shealy D, Mcdonough M, Scallon B, Moore MA, Vilcek J, Daddona P, Ghrayeb J (1992) Construction and initial characterization of a mouse-human chimeric anti-TNF antibody. Mol Immunol 30:1443–1453
Baert F, Noman M, Vermeire S, Van AG, D’ HG, Carbonez A, Rutgeerts P (2003) Influence of immunogenicity on the long-term efficacy of infliximab in Crohn's disease. N Engl J Med 348:601–608
Maser EA, Villela R, Silverberg MS, Greenberg GR (2006) Association of trough serum infliximab to clinical outcome after scheduled maintenance treatment for Crohn's disease. Clin Gastroenterol Hepatol 4:1248–1254
Farrell RJ, Alsahli M, Jeen YT, Falchuk KR, Peppercorn MA, Michetti P (2003) Intravenous hydrocortisone premedication reduces antibodies to infliximab in Crohn's disease: a randomized controlled trial. Gastroenterology 124:917–924
Regueiro M, Siemanowski B, Kip KE, Plevy S (2007) Infliximab dose intensification in Crohn's disease. Inflamm Bowel Dis 13:1093–1099
Hanauer SB, Wagner CL, Bala M, Mayer L, Travers S, Diamond RH, Olson A, Bao W, Rutgeerst P (2004) Incidence and importance of antibody responses to infliximab after maintenance or episodic treatment in Crohn's disease. Clin Gastroenterol Hepatol 2:542–553
Ben-Horin S, Yavzori M, Katz L, Kopylov U, Picard O, Fudim E, Coscas D, Bar-Meir S, Goldstein I, Chowers Y (2011) The immunogenic part of infliximab is the F(ab’)2, but measuring antibodies to the intact infliximab molecule is more clinically useful. Gut 60:41–48
Vermeire S, Noman M, Van AG, Baert F, D’Haens G, Rutgeerts P (2007) Effectiveness of concomitant immunosuppressive therapy in suppressing the formation of antibodies to infliximab in Crohn's disease. Gut 56:1226–1231
Ainsworth MA, Bendtzen K, Brynskov J (2008) Tumor necrosis factor-alpha binding capacity and anti-infliximab antibodies measured by fluid-phase radioimmunoassays as predictors of clinical efficacy of infliximab in Crohn's disease. Am J Gastroenterol 103:944–948
Seow CH, Newman A, Irwin SP, Steinhart AH, Silverberg MS, Greenberg GR (2010) Trough serum infliximab: a predictive factor of clinical outcome for infliximab treatment in acute ulcerative colitis. Gut 59:49–54
Radstake TR, Svenson M, Eijsbouts AM, van den Hoogen FH, Enevold C, Van Riel PL, Bendtzen K (2009) Formation of antibodies against infliximab and adalimumab strongly correlates with functional drug levels and clinical responses in rheumatoid arthritis. Ann Rheum Dis 68:1739–1745
Yanai H, Hanauer SB (2011) Assessing response and loss of response to biological therapies in IBD. Am J Gastroenterol 106:685–698
Afif W, Loftus EV Jr, Faubion WA, Kane SV, Bruining DH, Hanson KA, Sandborn WJ (2010) Clinical utility of measuring infliximab and human anti-chimeric antibody concentrations in patients with inflammatory bowel disease. Am J Gastroenterol 105:1133–1139
Miheller P, Kiss LS, Lorinczy K, Lakatos PL (2012) Anti-TNF trough levels and detection of antibodies to anti-TNF in inflammatory bowel disease: are they ready for everyday clinical use? Expert Opin Biol Ther 12:179–192
Corstjens PLAM, Li S, Zuiderwijk M, Kardos K, Abrams WR, Niedbala RS, Tanke HJ (2005) Infrared up-converting phophors for bioassays. IEE Proc Nanobiotechnol 152:62–72
Corstjens PLAM, de Dood CJ, van der Ploeg-van Schip, Wiesmeijer KC, Riuttamaki T, van Meijgaarden KE, Spencer JS, Tanke HJ, Ottenhoff THM, Geluk A (2011) Lateral flow assay for simultaneous detection of cellular- and humoral immune responses. Clin Biochem 44:1241–1246
Corstjens P, Zuiderwijk M, Brink A, Li S, Feindt H, Neidbala RS, Tanke H (2001) Use of up-converting phosphor reporters in lateral-flow assays to detect specific nucleic acid sequences: a rapid, sensitive DNA test to identify human papillomavirus type 16 infection. Clin Chem 47:1885–1893
Corstjens PL, Zuiderwijk M, Tanke HJ, van der Ploeg-van Schip JJ, Ottenhoff TH, Geluk A (2008) A user-friendly, highly sensitive assay to detect the IFN-gamma secretion by T cells. Clin Biochem 41:440–444
Niedbala RS, Feindt H, Kardos K, Vail T, Burton J, Bielska B et al (2001) Detection of analytes by immunoassay using up-converting phosphor technology. Anal Biochem 293:22–30
Mokkapati VK, Sam NR, Kardos K, Perez RJ, Guo M, Tanke HJ, Corstjens PL (2007) Evaluation of UPlink-RSV: prototype rapid antigen test for detection of respiratory syncytial virus infection. Ann N Y Acad Sci 1098:476–485
Wolbink GJ, Voskuyl AE, Lems WF, de Groot E, Nurmohamed MT, Tak PP, Dijkmans BAC, Aarden L (2005) Relationship between serum trough infliximab levels, pretreatment C reactive protein levels, and clinical response to infliximab treatment in patients with rheumatoid arthritis. Ann Rheum Dis 64:704–707
Rispens T, de Vrieze H, de Groot E, Wouters D, Stapel S, Wolbink GJ, Aarden LA (2012) Antibodies to constant domains of therapeutic monoclonal antibodies: anti-hinge antibodies in immunogenicity testing. J Immunol Methods 375:93–99
Corstjens PLAM, Chen ZY, Zuiderwijk M, Bau HH, Abrams WR, Malamud D, Niedbala RS, Tanke HJ (2007) Rapid assay format for multiplex detection of humoral immune responses to infectious disease pathogens (HIV, HCV, and TB). Ann N Y Acad Sci 1098:437–445
Baumgart DC, Sanborn WJ (2007) Inflammatory bowel disease: clinical aspects and established an evolving therapies. Lancet 369:1641–1657
Steenholdt C, Bendtzen K, Brynskov J, Thomsen OO, Ainsworth MA (2011) Cut-off levels and diagnostic accuracy of infliximab trough levels and anti-infliximab antibodies in Crohn's disease. Scand J Gastroenterol 46:310–318
Bendtzen K, Ainsworth M, Steenholdt C, Thomsen OO, Brynskov J (2009) Individual medicine in inflammatory bowel disease: monitoring bioavailability, pharmacokinetics and immunogenicity of anti-tumour necrosis factor-alpha antibodies. Scand J Gastroenterol 44:774–781
Ben-Horin S, Waterman M, Kopylov U, Yavzori M, Picard O, Fudim E, Awadi H, Weiss B, Chowers Y (2013) Addition of an immunomodulator to infliximab therapy eliminates antidrug antibodies in serum and restores clinical response of patients with inflammatory bowel disease. Clin Gastroenterol Hepatol 11:444–447
van der Valk ME, Mangen MJ, Leenders M, Dijkstra G, van Bodegraven AA, Fidder HH et al (2012) Healthcare costs of inflammatory bowel disease have shifted from hospitalisation and surgery towards anti-TNFalpha therapy: results from the COIN study. Gut. doi:10.1136/gutjnl-2012-303376
Wolbink GJ, Aarden LA, Dijkmans BA (2009) Dealing with immunogenicity of biologicals: assessment and clinical relevance. Curr Opin Rheumatol 21:211–215
Iregbu KC, Esfandiari J, Nnorom J, Sonibare SA, Uwaezuoke SN, Eze SO, Abdullahi N, Lawal AO, Durogbola BS (2011) Dual path platform HIV 1/2 assay: evaluation of a novel rapid test using oral fluids for HIV screening at the National Hospital in Abuja, Nigeria. Diagn Microbiol Infect Dis 69:405–409
Bruls DM, Evers TH, Kahlman JA, van Lankvelt PJ, Ovsyanko M, Pelssers EG (2009) Rapid integrated biosensor for multiplexed immunoassays based on actuated magnetic nanoparticles. Lab Chip 9:3504–3510
van der Marel S, Duijvestein M, Hardwick JC, van den Brink GR, Veenendaal R, Hommes DW, Fidder HH (2009) Quality of web-based information on inflammatory bowel diseases. Inflamm Bowel Dis 15:1891–1896
Acknowledgments
This study was financially supported by a research grant from Centocor BV, Leiden, the Netherlands and U.S. National Institute of Health grant UO1DE017855. We thank J.M. van der Zon and A.E. van de Meulen-de Jong (LUMC, Department of Gasteroenterolgy and Hepatology) for their help with the patient materials. We also thank D. van der Kleij (Sanquin, Diagnostic Services Division) for the ELISA data.
Author information
Authors and Affiliations
Corresponding author
Additional information
Paul L.A.M. Corstjens and Herma H. Fidder contributed equally to this work.
Rights and permissions
About this article
Cite this article
Corstjens, P.L.A.M., Fidder, H.H., Wiesmeijer, K.C. et al. A rapid assay for on-site monitoring of infliximab trough levels: a feasibility study. Anal Bioanal Chem 405, 7367–7375 (2013). https://doi.org/10.1007/s00216-013-7154-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-013-7154-0