Abstract
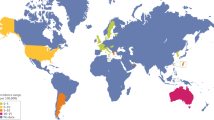
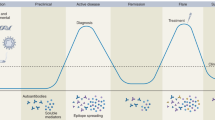
Systemic sclerosis is a complex autoimmune disease characterized by a chronic and frequently progressive course and by extensive patient-to-patient variability. Like other autoimmune diseases, systemic sclerosis occurs more frequently in women, with a peak of onset in the fifth decade of life. The exact cause of systemic sclerosis remains elusive but is likely to involve environmental factors in a genetically primed individual. Pathogenesis is dominated by vascular changes; evidence of autoimmunity with distinct autoantibodies and activation of both innate and adaptive immunity; and fibrosis of the skin and visceral organs that results in irreversible scarring and organ failure. Intractable progression of vascular and fibrotic organ damage accounts for the chronic morbidity and high mortality. Early and accurate diagnosis and classification might improve patient outcomes. Screening strategies facilitate timely recognition of life-threatening complications and initiation of targeted therapies to halt their progression. Effective treatments of organ-based complications are now within reach. Discovery of biomarkers — including autoantibodies that identify patient subsets at high risk for particular disease complications or rapid progression — is a research priority. Understanding the key pathogenetic pathways, cell types and mediators underlying disease manifestations opens the door for the development of targeted therapies with true disease-modifying potential. For an illustrated summary of this Primer, visit: http://go.nature.com/lchkcA
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 1 digital issues and online access to articles
$99.00 per year
only $99.00 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Nihtyanova, S. I. et al. Prediction of pulmonary complications and long-term survival in systemic sclerosis. Arthritis Rheumatol. 66, 1625–1635 (2014).
Domsic, R. T., Rodriguez-Reyna, T., Lucas, M., Fertig, N. & Medsger, T. A. Skin thickness progression rate: a predictor of mortality and early internal organ involvement in diffuse scleroderma. Ann. Rheum. Dis. 70, 104–109 (2011).
LeRoy, E. C. et al. Scleroderma (systemic sclerosis): classification, subsets and pathogenesis. J. Rheumatol. 15, 202–205 (1988).
Steen, V. D. Autoantibodies in systemic sclerosis. Semin. Arthritis Rheum. 35, 35–42 (2005).
Van den Hoogen, F. et al. 2013 classification criteria for systemic sclerosis: an American College of Rheumatology/European League Against Rheumatism Collaborative Initiative. Arthritis Rheum. 65, 2737–2747 (2013). This paper describes the revised classification criteria for systemic sclerosis.
Van den Hoogen, F. et al. 2013 classification criteria for systemic sclerosis: an American college of rheumatology/European league against rheumatism collaborative initiative. Ann. Rheum. Dis. 72, 1747–1755 (2013).
Barnes, J. & Mayes, M. D. Epidemiology of systemic sclerosis: incidence, prevalence, survival, risk factors, malignancy, and environmental triggers. Curr. Opin. Rheumatol. 24, 165–170 (2012).
Andréasson, K., Saxne, T., Bergknut, C., Hesselstrand, R. & Englund, M. Prevalence and incidence of systemic sclerosis in southern Sweden: population-based data with case ascertainment using the 1980 ARA criteria and the proposed ACR–EULAR classification criteria. Ann. Rheum. Dis. 73, 1788–1792 (2014). This paper describes prevalence and incidence estimations using the EULAR database and the revised classification criteria.
Elhai, M. et al. A gender gap in primary and secondary heart dysfunctions in systemic sclerosis: a EUSTAR prospective study. Ann. Rheum. Dis.http://dx.doi.org/10.1136/annrheumdis-2014-206386 (2014). This study highlights the gender gap in disease manifestations associated with systemic sclerosis.
Gelber, A. C. et al. Race and association with disease manifestations and mortality in scleroderma: a 20-year experience at the Johns Hopkins Scleroderma Center and review of the literature. Medicine 92, 191–205 (2013).
Elhai, M., Avouac, J., Kahan, A. & Allanore, Y. Systemic sclerosis at the crossroad of polyautoimmunity. Autoimmun. Rev. 12, 1052–1057 (2013).
Katsumoto, T. R., Whitfield, M. L. & Connolly, M. K. The pathogenesis of systemic sclerosis. Annu. Rev. Pathol. 6, 509–537 (2011).
Bhattacharyya, S., Wei, J. & Varga, J. Understanding fibrosis in systemic sclerosis: shifting paradigms, emerging opportunities. Nat. Rev. Rheumatol. 8, 42–54 (2012). This is an up-to-date comprehensive review on fibrotic-complication mechanisms of systemic sclerosis.
Trojanowska, M. Cellular and molecular aspects of vascular dysfunction in systemic sclerosis. Nat. Rev. Rheumatol. 6, 453–460 (2010).
Mayes, M. D. et al. ImmunoChIP analysis identifies multiple susceptibility loci for systemic sclerosis. Am. J. Hum. Genet. 94, 47–61 (2014).
Assassi, S. et al. Systemic sclerosis and lupus: points in an interferon-mediated continuum. Arthritis Rheum. 62, 589–598 (2010).
Kim, D. et al. Induction of interferon-α by scleroderma sera containing autoantibodies to topoisomerase I: association of higher interferon-α activity with lung fibrosis. Arthritis Rheum. 58, 2163–2173 (2008).
York, M. R. et al. A macrophage marker, Siglec-1, is increased on circulating monocytes in patients with systemic sclerosis and induced by type I interferons and Toll-like receptor agonists. Arthritis Rheum. 56, 1010–1020 (2007).
Chizzolini, C., Parel, Y., Scheja, A. & Dayer, J.-M. Polarized subsets of human T-helper cells induce distinct patterns of chemokine production by normal and systemic sclerosis dermal fibroblasts. Arthritis Res. Ther. 8, R10 (2006).
Brembilla, N. C. et al. TH17 cells favor inflammatory responses while inhibiting type I collagen deposition by dermal fibroblasts: differential effects in healthy and systemic sclerosis fibroblasts. Arthritis Res. Ther. 15, R151 (2013).
Shimizu, K. et al. Increased serum levels of soluble CD163 in patients with scleroderma. Clin. Rheumatol. 31, 1059–1064 (2012).
Christmann, R. B. et al. Association of Interferon- and transforming growth factor β-regulated genes and macrophage activation with systemic sclerosis-related progressive lung fibrosis. Arthritis Rheumatol. 66, 714–725 (2014). This paper highlights the role of innate immunity and macrophages in systemic sclerosis-associated interstitial lung disease.
Mathes, A. L. et al. Global chemokine expression in systemic sclerosis (SSc): CCL19 expression correlates with vascular inflammation in SSc skin. Ann. Rheum. Dis. 73, 1864–1872 (2014).
Mathai, S. K. et al. Circulating monocytes from systemic sclerosis patients with interstitial lung disease show an enhanced profibrotic phenotype. Lab. Invest. 90, 812–823 (2010).
O'Reilly, S., Hügle, T. & van Laar, J. M. T cells in systemic sclerosis: a reappraisal. Rheumatology 51, 1540–1549 (2012).
O'Reilly, S. Role of interleukin-13 in fibrosis, particularly systemic sclerosis. Biofactors 39, 593–596 (2013).
Khan, K. et al. Clinical and pathological significance of interleukin 6 overexpression in systemic sclerosis. Ann. Rheum. Dis. 71, 1235–1242 (2012).
Kitaba, S. et al. Blockade of interleukin-6 receptor alleviates disease in mouse model of scleroderma. Am. J. Pathol. 180, 165–176 (2012).
Yoshizaki, A. et al. Immunization with DNA topoisomerase I and Freund's complete adjuvant induces skin and lung fibrosis and autoimmunity via interleukin-6 signaling. Arthritis Rheum. 63, 3575–3585 (2011).
Bandinelli, F. et al. CCL2, CCL3 and CCL5 chemokines in systemic sclerosis: the correlation with SSc clinical features and the effect of prostaglandin E1 treatment. Clin. Exp. Rheumatol. 30, S44–49 (2012).
Hasegawa, M. et al. Serum chemokine levels as prognostic markers in patients with early systemic sclerosis: a multicenter, prospective, observational study. Mod. Rheumatol. 23, 1076–1084 (2013).
Tiev, K. P. et al. Serum CC chemokine ligand-18 predicts lung disease worsening in systemic sclerosis. Eur. Respir. J. 38, 1355–1360 (2011).
Van Bon, L. et al. Proteome-wide analysis and CXCL4 as a biomarker in systemic sclerosis. N. Engl. J. Med. 370, 433–443 (2014). This paper describes plasmacytoid dendritic cells as important mediators of fibrosis.
Dragun, D., Distler, J. H. W., Riemekasten, G. & Distler, O. Stimulatory autoantibodies to platelet-derived growth factor receptors in systemic sclerosis: what functional autoimmunity could learn from receptor biology. Arthritis Rheum. 60, 907–911 (2009).
Baroni, S. S. et al. Stimulatory autoantibodies to the PDGF receptor in systemic sclerosis. N. Engl. J. Med. 354, 2667–2676 (2006).
Kill, A. et al. Autoantibodies to angiotensin and endothelin receptors in systemic sclerosis induce cellular and systemic events associated with disease pathogenesis. Arthritis Res. Ther. 16, R29 (2014).
Castelino, F. V. & Varga, J. Emerging cellular and molecular targets in fibrosis: implications for scleroderma pathogenesis and targeted therapy. Curr. Opin. Rheumatol. 26, 607–614 (2014).
Hinz, B. et al. Recent developments in myofibroblast biology: paradigms for connective tissue remodeling. Am. J. Pathol. 180, 1340–1355 (2012).
Marangoni, R. G. et al. Myofibroblasts in cutaneous fibrosis originate from adiponectin-positive intradermal progenitors. Arthritis Rheumatol.http://dx.doi.org/10.1002/art.38990 (2014). This paper describes the adipogenic progenitor cells as precursors of myofibroblasts in skin lesions associated with systemic sclerosis.
Ho, Y. Y., Lagares, D., Tager, A. M. & Kapoor, M. Fibrosis — a lethal component of systemic sclerosis. Nat. Rev. Rheumatol. 10, 390–402 (2014).
Bhattacharyya, S. et al. FibronectinEDA promotes chronic cutaneous fibrosis through Toll-like receptor signaling. Sci. Transl. Med. 6, 232ra50 (2014).
Varga, J. & Pasche, B. Transforming growth factor β as a therapeutic target in systemic sclerosis. Nat. Rev. Rheumatol. 5, 200–206 (2009).
Asano, Y., Ihn, H., Yamane, K., Kubo, M. & Tamaki, K. Impaired SMAD7–SMURF-mediated negative regulation of TGF-β signaling in scleroderma fibroblasts. J. Clin. Invest. 113, 253–264 (2004).
Zhou, F. et al. Nuclear receptor NR4A1 promotes breast cancer invasion and metastasis by activating TGF-β signalling. Nat. Commun. 5, 3388 (2014).
Wong, V. W. et al. Focal adhesion kinase links mechanical force to skin fibrosis via inflammatory signaling. Nature Med. 18, 148–152 (2012).
Abraham, D. Connective tissue growth factor: growth factor, matricellular organizer, fibrotic biomarker or molecular target for anti-fibrotic therapy in SSc? Rheumatology 47, (Suppl. 5), v8–v9 (2008).
Leask, A., Denton, C. P. & Abraham, D. J. Insights into the molecular mechanism of chronic fibrosis: the role of connective tissue growth factor in scleroderma. J. Invest. Dermatol. 122, 1–6 (2004).
Trojanowska, M. Role of PDGF in fibrotic diseases and systemic sclerosis. Rheumatology 47, (Suppl. 5), v2–v4 (2008).
Olson, L. E. & Soriano, P. Increased PDGFRα activation disrupts connective tissue development and drives systemic fibrosis. Dev. Cell 16, 303–313 (2009).
Svegliati, S. et al. Oxidative DNA damage induces the ATM-mediated transcriptional suppression of the WNT inhibitor WIF-1 in systemic sclerosis and fibrosis. Sci. Signal. 7, ra84 (2014). This study implicates oxidative stress and WNT signalling as key drivers in tissue damage and fibrosis.
Beyer, C. et al. Blockade of canonical WNT signalling ameliorates experimental dermal fibrosis. Ann. Rheum. Dis. 72, 1255–1258 (2013).
Lam, A. P. et al. WNT coreceptor Lrp5 is a driver of idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 190, 185–195 (2014).
Wei, J. et al. WNT/β-catenin signaling is hyperactivated in systemic sclerosis and induces Smad-dependent fibrotic responses in mesenchymal cells. Arthritis Rheum. 64, 2734–2745 (2012).
Arnett, F. C. et al. Familial occurrence frequencies and relative risks for systemic sclerosis (scleroderma) in three United States cohorts. Arthritis Rheum. 44, 1359–1362 (2001).
Frech, T. et al. Heritability of vasculopathy, autoimmune disease, and fibrosis in systemic sclerosis: a population-based study. Arthritis Rheum. 62, 2109–2116 (2010).
Varga, J. & Hinchcliff, M. Connective tissue diseases: systemic sclerosis: beyond limited and diffuse subsets? Nat. Rev. Rheumatol. 10, 200–202 (2014).
Altorok, N., Almeshal, N., Wang, Y. & Kahaleh, B. Epigenetics, the holy grail in the pathogenesis of systemic sclerosis. Rheumatologyhttp://dx.doi.org/10.1093/rheumatology/keu155 (2014).
Broen, J. C. A., Radstake, T. R. D. J. & Rossato, M. The role of genetics and epigenetics in the pathogenesis of systemic sclerosis. Nat. Rev. Rheumatol. 10, 671–681 (2014).
Altorok, N., Tsou, P.-S., Coit, P., Khanna, D. & Sawalha, A. H. Genome-wide DNA methylation analysis in dermal fibroblasts from patients with diffuse and limited systemic sclerosis reveals common and subset-specific DNA methylation aberrancies. Ann. Rheum. Dis.http://dx.doi.org/10.1136/annrheumdis-2014-205303 (2014).
Noda, S. et al. Simultaneous downregulation of KLF5 and Fli1 is a key feature underlying systemic sclerosis. Nat. Commun. 5, 5797 (2014).
Dees, C. et al. The WNT antagonists DKK1 and SFRP1 are downregulated by promoter hypermethylation in systemic sclerosis. Ann. Rheum. Dis. 73, 1232–1239 (2014).
Wang, Y., Fan, P.-S. & Kahaleh, B. Association between enhanced type I collagen expression and epigenetic repression of the FLI1 gene in scleroderma fibroblasts. Arthritis Rheum. 54, 2271–2279 (2006).
Lei, W. et al. Abnormal DNA methylation in CD4+ T cells from patients with systemic lupus erythematosus, systemic sclerosis, and dermatomyositis. Scand. J. Rheumatol. 38, 369–374 (2009).
Wang, Y. Y. et al. DNA hypermethylation of the forkhead box protein 3 (FOXP3) promoter in CD4+ T cells of patients with systemic sclerosis. Br. J. Dermatol. 171, 39–47 (2014).
Ghosh, A. K., Mori, Y., Dowling, E. & Varga, J. Trichostatin A blocks TGF-β-induced collagen gene expression in skin fibroblasts: involvement of Sp1. Biochem. Biophys. Res. Commun. 354, 420–426 (2007).
Huber, L. C. et al. Trichostatin A prevents the accumulation of extracellular matrix in a mouse model of bleomycin-induced skin fibrosis. Arthritis Rheum. 56, 2755–2764 (2007).
Ghosh, A. K. et al. p300 is elevated in systemic sclerosis and its expression is positively regulated by TGF-β: epigenetic feed-forward amplification of fibrosis. J. Invest. Dermatol. 133, 1302–1310 (2013).
Zhu, H. et al. MicroRNA-21 in scleroderma fibrosis and its function in TGF-β-regulated fibrosis-related genes expression. J. Clin. Immunol. 33, 1100–1109 (2013).
Bhattacharyya, S. et al. Toll-like receptor 4 signaling augments transforming growth factor-β responses: a novel mechanism for maintaining and amplifying fibrosis in scleroderma. Am. J. Pathol. 182, 192–205 (2013).
Maurer, B. et al. MicroRNA-29, a key regulator of collagen expression in systemic sclerosis. Arthritis Rheum. 62, 1733–1743 (2010).
Chaudhary, P. et al. Cigarette smoking is not a risk factor for systemic sclerosis. Arthritis Rheum. 63, 3098–3102 (2011).
Hissaria, P. et al. Survival in scleroderma: results from the population-based South Australian Register. Intern. Med. J. 41, 381–390 (2011).
Dospinescu, P., Jones, G. T. & Basu, N. Environmental risk factors in systemic sclerosis. Curr. Opin. Rheumatol. 25, 179–183 (2013).
Lunardi, C. et al. Antibodies against human cytomegalovirus in the pathogenesis of systemic sclerosis: a gene array approach. PLoS Med. 3, e2 (2006).
Farina, A. et al. Epstein–Barr virus infection induces aberrant TLR activation pathway and fibroblast–myofibroblast conversion in scleroderma. J. Invest. Dermatol. 134, 954–964 (2014). This paper describes the potential pathogenic role of latent virus infection in triggering systemic sclerosis.
[No authors listed]. Preliminary criteria for the classification of systemic sclerosis (scleroderma). Subcommittee for scleroderma criteria of the American Rheumatism Association Diagnostic and Therapeutic Criteria Committee. Arthritis Rheum. 23, 581–590 (1980).
Lonzetti, L. S. et al. Updating the American College of Rheumatology preliminary classification criteria for systemic sclerosis: addition of severe nailfold capillaroscopy abnormalities markedly increases the sensitivity for limited scleroderma. Arthritis Rheum. 44, 735–736 (2001).
Alhajeri, H. et al. The 2013 ACR/EULAR Classification Criteria for Systemic Sclerosis out-perform the 1980 Criteria. Data from the Canadian Scleroderma Research Group. Arthritis Care Res.http://dx.doi.org/10.1002/acr.22451 (2015).
Jordan, S., Maurer, B., Toniolo, M., Michel, B. & Distler, O. Performance of the new ACR/EULAR classification criteria for systemic sclerosis in clinical practice. Rheumatology (Oxford)http://dx.doi.org/10.1093/rheumatology/keu530 (2015).
Avouac, J. et al. Preliminary criteria for the Very Early Diagnosis of Systemic Sclerosis: results of a Delphi Consensus Study from EULAR Scleroderma Trials and Research Group. Ann. Rheum. Dis. 70, 476–481 (2011).
Minier, T. et al. Preliminary analysis of the Very Early Diagnosis of Systemic Sclerosis (VEDOSS) EUSTAR multicentre study: evidence for puffy fingers as a pivotal sign for suspicion of systemic sclerosis. Ann. Rheum. Dis. 73, 2087–2093 (2013).
Koenig, M. et al. Autoantibodies and microvascular damage are independent predictive factors for the progression of Raynaud's phenomenon to systemic sclerosis: a twenty-year prospective study of 586 patients, with validation of proposed criteria for early systemic sclerosi. Arthritis Rheum. 58, 3902–3912 (2008). This paper reinforces that Raynaud phenomenon precedes and can predict development of systemic sclerosis in some cases.
Valentini, G. et al. Early systemic sclerosis: analysis of the disease course in patients with marker autoantibody and/or capillaroscopic positivity. Arthritis Care Res. 66, 1520–1527 (2014).
Bartelink, M. L., Wollersheim, H., van de Lisdonk, E., Spruijt, R. & van Weel, C. Prevalence of Raynaud's phenomenon. Neth. J. Med. 41, 149–152 (1992).
Valentini, G. et al. Early systemic sclerosis: assessment of clinical and pre-clinical organ involvement in patients with different disease features. Rheumatology 50, 317–323 (2011).
Lepri, G. et al. Evidence for oesophageal and anorectal involvement in very early systemic sclerosis (VEDOSS): report from a single VEDOSS/EUSTAR centre. Ann. Rheum. Dis. 74, 124–128 (2015).
Steen, V. D. & Medsger, T. A. Severe organ involvement in systemic sclerosis with diffuse scleroderma. Arthritis Rheum. 43, 2437–2444 (2000). This paper describes predictors of severe organ damage in systemic sclerosis.
Humbert, M. et al. Screening for pulmonary arterial hypertension in patients with systemic sclerosis: clinical characteristics at diagnosis and long-term survival. Arthritis Rheum. 63, 3522–3530 (2011).
Galiè, N. et al. Treatment of patients with mildly symptomatic pulmonary arterial hypertension with bosentan (EARLY study): a double-blind, randomised controlled trial. Lancet 371, 2093–2100 (2008).
Furst, D. E. et al. The modified Rodnan skin score is an accurate reflection of skin biopsy thickness in systemic sclerosis. J. Rheumatol. 25, 84–88 (1998).
Clements, P. et al. Inter and intraobserver variability of total skin thickness score (modified Rodnan TSS) in systemic sclerosis. J. Rheumatol. 22, 1281–1285 (1995).
Merkel, P. A. et al. Patterns and predictors of change in outcome measures in clinical trials in scleroderma: an individual patient meta-analysis of 629 subjects with diffuse cutaneous systemic sclerosis. Arthritis Rheum. 64, 3420–3429 (2012).
Maurer, B. et al. Prediction of worsening of skin fibrosis in patients with diffuse cutaneous systemic sclerosis using the EUSTAR database. Ann. Rheum. Dis.http://dx.doi.org/10.1136/annrheumdis-2014-205226 (2014).
Lefèvre, G. et al. Survival and prognostic factors in systemic sclerosis-associated pulmonary hypertension: a systematic review and meta-analysis. Arthritis Rheum. 65, 2412–2423 (2013).
Shahane, A. Pulmonary hypertension in rheumatic diseases: epidemiology and pathogenesis. Rheumatol. Int. 33, 1655–1667 (2013).
Shah, A. A., Wigley, F. M. & Hummers, L. K. Telangiectases in scleroderma: a potential clinical marker of pulmonary arterial hypertension. J. Rheumatol. 37, 98–104 (2010).
Galiè, N. et al. Guidelines for the diagnosis and treatment of pulmonary hypertension: the Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the Internat. Eur. Heart J. 30, 2493–2537 (2009).
McLaughlin, V. V. et al. ACCF/AHA 2009 expert consensus document on pulmonary hypertension: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association: developed in collaboration with the American College. Circulation 119, 2250–2294 (2009).
Avouac, J. et al. Expert consensus for performing right heart catheterisation for suspected pulmonary arterial hypertension in systemic sclerosis: a Delphi consensus study with cluster analysis. Ann. Rheum. Dis. 73, 191–197 (2014).
Khanna, D. et al. Recommendations for screening and detection of connective tissue disease-associated pulmonary arterial hypertension. Arthritis Rheum. 65, 3194–3201 (2013).
Meune, C. et al. Prediction of pulmonary hypertension related to systemic sclerosis by an index based on simple clinical observations. Arthritis Rheum. 63, 2790–2796 (2011).
Coghlan, J. G. et al. Evidence-based detection of pulmonary arterial hypertension in systemic sclerosis: the DETECT study. Ann. Rheum. Dis. 73, 1340–1349 (2014).
Winstone, T. A. et al. Predictors of mortality and progression in scleroderma-associated interstitial lung disease: a systematic review. Chest 146, 422–436 (2014).
Suliman, Y. A. et al. FRI0377 High rate of false negatives in the early detection of interstitial lung disease associated with systemic sclerosis by pulmonary function tests. Ann. Rheum. Dis. 72, A500–A501 (2014).
Goldin, J. G. et al. High-resolution CT scan findings in patients with symptomatic scleroderma-related interstitial lung disease. Chest 134, 358–367 (2008).
Herzog, E. L. et al. Review: interstitial lung disease associated with systemic sclerosis and idiopathic pulmonary fibrosis: how similar and distinct?. Arthritis Rheumatol. 66, 1967–1978 (2014). This review highlights similarities and differences among systemic sclerosis-associated interstitial lung disease and idiopathic pulmonary fibrosis.
Barskova, T. et al. Lung ultrasound for the screening of interstitial lung disease in very early systemic sclerosis. Ann. Rheum. Dis. 72, 390–395 (2013).
Frauenfelder, T. et al. Screening for interstitial lung disease in systemic sclerosis: performance of high-resolution CT with limited number of slices: a prospective study. Ann. Rheum. Dis. 73, 2069–2073 (2014).
Winklehner, A. et al. Screening for interstitial lung disease in systemic sclerosis: the diagnostic accuracy of HRCT image series with high increment and reduced number of slices. Ann. Rheum. Dis. 71, 549–552 (2012).
Moore, O. A. et al. Extent of disease on high-resolution computed tomography lung is a predictor of decline and mortality in systemic sclerosis-related interstitial lung disease. Rheumatol. (Oxford). 52, 155–160 (2013).
Khanna, D. et al. Clinical course of lung physiology in patients with scleroderma and interstitial lung disease: analysis of the Scleroderma Lung Study Placebo Group. Arthritis Rheum. 63, 3078–3085 (2011).
Goh, N. S. L. et al. Interstitial lung disease in systemic sclerosis: a simple staging system. Am. J. Respir. Crit. Care Med. 177, 1248–1254 (2008). This paper describes a simple and validated approach for assessing lung fibrosis and predicting progression of lung disease in systemic sclerosis.
Perera, A. et al. Clinical subsets, skin thickness progression rate, and serum antibody levels in systemic sclerosis patients with anti-topoisomerase I antibody. Arthritis Rheum. 56, 2740–2746 (2007).
Steen, V., Domsic, R. T., Lucas, M., Fertig, N. & Medsger, T. A. A clinical and serologic comparison of African American and caucasian patients with systemic sclerosis. Arthritis Rheum. 64, 2986–2994 (2012).
Sebastiani, M. et al. Capillaroscopic skin ulcer risk index: a new prognostic tool for digital skin ulcer development in systemic sclerosis patients. Arthritis Rheum. 61, 688–694 (2009).
Sebastiani, M. et al. Predictive role of capillaroscopic skin ulcer risk index in systemic sclerosis: a multicentre validation study. Ann. Rheum. Dis. 71, 67–70 (2012).
Shah, A. A. & Wigley, F. M. My approach to the treatment of scleroderma. Mayo Clin. Proc. 88, 377–393 (2013).
Simms, R. W. & Korn, J. H. in Rheumatology and the Kidney (eds Adu, D., Emery, P. & Madaio, M. ) 275–293 (Oxford Univ. Press, 2001).
Ostojić, P. Damjanov N. Pavlov-Dolijanovic S. & Radunović, G. Peripheral vasculopathy in patients with systemic sclerosis: difference in limited and diffuse subset of disease. Clin. Hemorheol. Microcirc. 31, 281–285 (2004).
Burt, R. K. et al. Autologous non-myeloablative haemopoietic stem-cell transplantation compared with pulse cyclophosphamide once per month for systemic sclerosis (ASSIST): an open-label, randomised Phase 2 trial. Lancet 378, 498–506 (2011). This randomized clinical trial shows positive effects of treating systemic sclerosis with HSC transplantation.
Van Laar, J. M. et al. Autologous hematopoietic stem cell transplantation versus intravenous pulse cyclophosphamide in diffuse cutaneous systemic sclerosis: a randomized clinical trial. JAMA 311, 2490–2498 (2014).
Schachna, L. et al. Lung transplantation in scleroderma compared with idiopathic pulmonary fibrosis and idiopathic pulmonary arterial hypertension. Arthritis Rheum. 54, 3954–3961 (2006).
Tashkin, D. P. et al. Cyclophosphamide versus placebo in scleroderma lung disease. N. Engl. J. Med. 354, 2655–2666 (2006). This paper describes results from a randomized clinical trial of cyclophosphamide.
Tashkin, D. P. et al. Effects of 1-year treatment with cyclophosphamide on outcomes at 2 years in scleroderma lung disease. Am. J. Respir. Crit. Care Med. 176, 1026–1034 (2007).
Hoyles, R. K. et al. A multicenter, prospective, randomized, double-blind, placebo-controlled trial of corticosteroids and intravenous cyclophosphamide followed by oral azathioprine for the treatment of pulmonary fibrosis in scleroderma. Arthritis Rheum. 54, 3962–3970 (2006).
Badesch, D. B. et al. Continuous intravenous epoprostenol for pulmonary hypertension due to the scleroderma spectrum of disease. A randomized, controlled trial. Ann. Intern. Med. 132, 425–434 (2000).
Chaisson, N. F. & Hassoun, P. M. Systemic sclerosis-associated pulmonary arterial hypertension. Chest 144, 1346–1356 (2013).
Pulido, T. et al. Macitentan and morbidity and mortality in pulmonary arterial hypertension. N. Engl. J. Med. 369, 809–818 (2013).
Buckley, M. S., Staib, R. L. & Wicks, L. M. Combination therapy in the management of pulmonary arterial hypertension. Int. J. Clin. Pract. 67, (Suppl. S179), 13–23 (2013).
Badesch, D. B. et al. Longterm survival among patients with scleroderma-associated pulmonary arterial hypertension treated with intravenous epoprostenol. J. Rheumatol. 36, 2244–2249 (2009).
Amjadi, S. et al. Course of the modified Rodnan skin thickness score in systemic sclerosis clinical trials: analysis of three large multicenter, double-blind, randomized controlled trials. Arthritis Rheum. 60, 2490–2498 (2009).
Pope, J. E. et al. A randomized, controlled trial of methotrexate versus placebo in early diffuse scleroderma. Arthritis Rheum. 44, 1351–1358 (2001).
Penn, H. & Denton, C. P. Diagnosis, management and prevention of scleroderma renal disease. Curr. Opin. Rheumatol. 20, 692–696 (2008).
Steen, V. D. Scleroderma renal crisis. Rheum. Dis. Clin. North Am. 22, 861–878 (1996).
Mouthon, L., Bussone, G., Berezné, A., Noël, L.-H. & Guillevin, L. Scleroderma renal crisis. J. Rheumatol. 41, 1040–1048 (2014).
Steen, V. D. Scleroderma renal crisis. Rheum. Dis. Clin. North Am. 29, 315–333 (2003).
Hudson, M., Baron, M., Tatibouet, S., Furst, D. E. & Khanna, D. Exposure to ACE inhibitors prior to the onset of scleroderma renal crisis — results from the International Scleroderma Renal Crisis Survey. Semin. Arthritis Rheum. 43, 666–672 (2014).
Hachulla, E. et al. Natural history of ischemic digital ulcers in systemic sclerosis: single-center retrospective longitudinal study. J. Rheumatol. 34, 2423–2430 (2007).
Pope, J. Measures of systemic sclerosis (scleroderma): Health Assessment Questionnaire (HAQ) and Scleroderma HAQ (SHAQ), physician- and patient-rated global assessments, Symptom Burden Index (SBI), University of California, Los Angeles, Scleroderma Clinical Trials. Arthritis Care Res. 63, (Suppl. 1), S98–S111 (2011).
Chung, L. Therapeutic options for digital ulcers in patients with systemic sclerosis. J. Dtsch. Dermatol. Ges. 5, 460–465 (2007).
Tingey, T., Shu, J., Smuczek, J. & Pope, J. Meta-analysis of healing and prevention of digital ulcers in systemic sclerosis. Arthritis Care Res. 65, 1460–1471 (2013).
Korn, J. H. et al. Digital ulcers in systemic sclerosis: prevention by treatment with bosentan, an oral endothelin receptor antagonist. Arthritis Rheum. 50, 3985–3993 (2004).
Matucci-Cerinic, M. et al. Bosentan treatment of digital ulcers related to systemic sclerosis: results from the RAPIDS-2 randomised, double-blind, placebo-controlled trial. Ann. Rheum. Dis. 70, 32–38 (2011).
Asano, Y. & Trojanowska, M. Phosphorylation of Fli1 at threonine 312 by protein kinase C delta promotes its interaction with p300/CREB-binding protein-associated factor and subsequent acetylation in response to transforming growth factor beta. Mol. Cell. Biol. 29, 1882–1894 (2009).
Steen, V. D. & Medsger, T. A. Case–control study of corticosteroids and other drugs that either precipitate or protect from the development of scleroderma renal crisis. Arthritis Rheum. 41, 1613–1619 (1998). This study shows that glucocorticoids are a risk factor for the development of renal crisis.
Elhai, M. et al. Outcomes of patients with systemic sclerosis-associated polyarthritis and myopathy treated with tocilizumab or abatacept: a EUSTAR observational study. Ann. Rheum. Dis. 72, 1217–1220 (2013).
Domsic, R., Fasanella, K. & Bielefeldt, K. Gastrointestinal manifestations of systemic sclerosis. Dig. Dis. Sci. 53, 1163–1174 (2008).
Jaovisidha, K., Csuka, M. E., Almagro, U. A. & Soergel, K. H. Severe gastrointestinal involvement in systemic sclerosis: report of five cases and review of the literature. Semin. Arthritis Rheum. 34, 689–702 (2005).
Watson, M., Hally, R. J., McCue, P. A., Varga, J. & Jiménez, S. A. Gastric antral vascular ectasia (watermelon stomach) in patients with systemic sclerosis. Arthritis Rheum. 39, 341–346 (1996).
Marie, I., Duparc, F., Janvresse, A., Levesque, H. & Courtois, H. Tumoral calcinosis in systemic sclerosis. Clin. Exp. Rheumatol. 22, 269 (2004).
Taki, H. & Tobe, K. Tumoral calcinosis in systemic sclerosis. Joint. Bone. Spine 80, 99 (2013).
Gutierrez, A. & Wetter, D. A. Calcinosis cutis in autoimmune connective tissue diseases. Dermatol. Ther. 25, 195–206 (2012).
Chung, L. et al. Validation of a novel radiographic scoring system for calcinosis affecting the hands of patients with systemic sclerosis. Arthritis Care Res. 67, 425–430 (2015).
Al-Dhaher, F. F., Pope, J. E. & Ouimet, J. M. Determinants of morbidity and mortality of systemic sclerosis in Canada. Semin. Arthritis Rheum. 39, 269–277 (2010).
Hudson, M. et al. Health-related quality of life in systemic sclerosis: a systematic review. Arthritis Rheum. 61, 1112–1120 (2009).
Cole, J. C. et al. Single-factor scoring validation for the Health Assessment Questionnaire- Disability Index (HAQ-DI) in patients with systemic sclerosis and comparison with early rheumatoid arthritis patients. Qual. Life Res. 15, 1383–1394 (2006).
Sekhon, S., Pope, J. & Baron, M. The minimally important difference in clinical practice for patient-centered outcomes including health assessment questionnaire, fatigue, pain, sleep, global visual analog scale, and SF-36 in scleroderma. J. Rheumatol. 37, 591–598 (2010). This study defines the minimally important differences in patient-reported outcomes.
El-Baalbaki, G., Lober, J., Hudson, M., Baron, M. & Thombs, B. D. Measuring pain in systemic sclerosis: comparison of the short-form McGill Pain Questionnaire versus a single-item measure of pain. J. Rheumatol. 38, 2581–2587 (2011).
Milette, K. et al. Clinical correlates of sleep problems in systemic sclerosis: the prominent role of pain. Rheumatology 50, 921–925 (2011).
Schnitzer, M., Hudson, M., Baron, M. & Steele, R. Disability in systemic sclerosis — a longitudinal observational study. J. Rheumatol. 38, 685–692 (2011).
Schieir, O. et al. Prevalence, severity, and clinical correlates of pain in patients with systemic sclerosis. Arthritis Care Res. 62, 409–417 (2010).
El-Baalbaki, G. et al. Association of pruritus with quality of life and disability in systemic sclerosis. Arthritis Care Res. 62, 1489–1495 (2010).
Hudson, M., Steele, R., Lu, Y., Thombs, B. D. & Baron, M. Work disability in systemic sclerosis. J. Rheumatol. 36, 2481–2486 (2009).
Hong, P., Pope, J. E., Ouimet, J. M., Rullan, E. & Seibold, J. R. Erectile dysfunction associated with scleroderma: a case–control study of men with scleroderma and rheumatoid arthritis. J. Rheumatol. 31, 508–513 (2004).
Ouimet, J. M., Pope, J. E., Gutmanis, I. & Koval, J. Work disability in scleroderma is greater than in rheumatoid arthritis and is predicted by high HAQ scores. Open Rheumatol. J. 2, 44–52 (2008).
Bassel, M. et al. Frequency and impact of symptoms experienced by patients with systemic sclerosis: results from a Canadian National Survey. Rheumatology 50, 762–767 (2011).
Khanna, D. et al. Reliability and validity of the University of California, Los Angeles Scleroderma Clinical Trial Consortium Gastrointestinal Tract Instrument. Arthritis Rheum. 61, 1257–1263 (2009).
Baron, M., Hudson, M., Steele, R. & Lo, E. Validation of the UCLA Scleroderma Clinica Trial Gastrointestinal Tract Instrument version 2.0 for systemic sclerosis. J. Rheumatol. 38, 1925–1930 (2011).
Muangchan, C., Baron, M. & Pope, J. The 15% rule in scleroderma: the frequency of severe organ complications in systemic sclerosis. A systematic review. J. Rheumatol. 40, 1545–1556 (2013).
Onishi, A., Sugiyama, D., Kumagai, S. & Morinobu, A. Cancer incidence in systemic sclerosis: meta-analysis of population-based cohort studies. Arthritis Rheum. 65, 1913–1921 (2013).
Shah, A. A., Rosen, A., Hummers, L., Wigley, F. & Casciola-Rosen, L. Close temporal relationship between onset of cancer and scleroderma in patients with RNA polymerase I/III antibodies. Arthritis Rheum. 62, 2787–2795 (2010). This seminal study provides potential mechanistic links between cancer and systemic sclerosis. Its clinical and biological relevance might provide future insights into aetiopathogenesis of dsSSc.
Moinzadeh, P. et al. Association of anti-RNA polymerase III autoantibodies and cancer in scleroderma. Arthritis Res. Ther. 16, R53 (2014).
Joseph, C. G. et al. Association of the autoimmune disease scleroderma with an immunologic response to cancer. Science 343, 152–157 (2014).
Malcarne, V. L., Fox, R. S., Mills, S. D. & Gholizadeh, S. Psychosocial aspects of systemic sclerosis. Curr. Opin. Rheumatol. 25, 707–713 (2013).
Thombs, B. D. et al. Psychological health and well-being in systemic sclerosis: State of the science and consensus research agenda. Arthritis Care Res. 62, 1181–1189 (2010). This is a review of quality-of-life studies in systemic sclerosis.
Jewett, L. R., Razykov, I., Hudson, M., Baron, M. & Thombs, B. D. Prevalence of current, 12-month and lifetime major depressive disorder among patients with systemic sclerosis. Rheumatology 52, 669–675 (2013).
Thombs, B. D., Jewett, L. R., Kwakkenbos, L., Hudson, M. & Baron, M. Major depression diagnoses are often transient among patients with systemic sclerosis: baseline and 1-month follow-up. Arthritis Care Res. 67, 411–416 (2015).
Jewett, L. R. et al. Development and validation of the brief-satisfaction with appearance scale for systemic sclerosis. Arthritis Care Res. 62, 1779–1786 (2010).
Kwakkenbos, L. et al. The Scleroderma Patient-centered Intervention Network (SPIN) Cohort: protocol for a cohort multiple randomised controlled trial (cmRCT) design to support trials of psychosocial and rehabilitation interventions in a rare disease context. BMJ Open 3, e003563 (2013).
Khanna, D. et al. Twenty-two points to consider for clinical trials in systemic sclerosis, based on EULAR standards. Rheumatology 54, 144–151 (2015). This paper provides detailed expert guidance for clinical trial design in systemic sclerosis, which will underpin the validation of emerging candidate therapies and accelerate therapeutic progress.
Domsic, R. T. et al. Derivation and validation of a prediction rule for two-year mortality in early diffuse cutaneous systemic sclerosis. Arthritis Rheumatol. 66, 1616–1624 (2014).
Wells, A. U. & Denton, C. P. Interstitial lung disease in connective tissue disease-mechanisms and management. Nat. Rev. Rheumatol. 10, 728–739 (2014).
Nihtyanova, S. I. et al. Improved survival in systemic sclerosis is associated with better ascertainment of internal organ disease: a retrospective cohort study. QJM 103, 109–115 (2010).
Beyer, C., Schett, G., Distler, O. & Distler, J. H. W. Animal models of systemic sclerosis: prospects and limitations. Arthritis Rheum. 62, 2831–2844 (2010).
Derrett-Smith, E. C. et al. Endothelial injury in a transforming growth factor β-dependent mouse model of scleroderma induces pulmonary arterial hypertension. Arthritis Rheum. 65, 2928–2939 (2013).
Quillinan, N. P., McIntosh, D., Vernes, J., Haq, S. & Denton, C. P. Treatment of diffuse systemic sclerosis with hyperimmune caprine serum (AIMSPRO): a phase II double-blind placebo-controlled trial. Ann. Rheum. Dis. 73, 56–61 (2014). This study is the first in recent years to test a novel biological therapy and to be able to differentiate between active treatment and placebo. This underscores the critical importance of having a placebo arm even in very small Phase I/II studies and will help in the design of future trials.
Steen, V. D. & Medsger, T. A. Changes in causes of death in systemic sclerosis, 1972–2002. Ann. Rheum. Dis. 66, 940–944 (2007).
Denton, C. P. & Ong, V. H. Targeted therapies for systemic sclerosis. Nat. Rev. Rheumatol. 9, 451–464 (2013). This is a review of current and emerging therapies and their rationale.
Asano, Y. & Trojanowska, M. Fli1 represses transcription of the human α2(I) collagen gene by recruitment of the HDAC1/p300 complex. PLoS ONE 8, e74930 (2013).
Koinuma, D. et al. Chromatin immunoprecipitation on microarray analysis of Smad2/3 binding sites reveals roles of ETS1 and TFAP2A in transforming growth factor β signaling. Mol. Cell. Biol. 29, 172–186 (2009).
Van Beek, J. P., Kennedy, L., Rockel, J. S., Bernier, S. M. & Leask, A. The induction of CCN2 by TGFβ1 involves Ets-1. Arthritis Res. Ther. 8, R36 (2006).
Ghosh, A. K. et al. Disruption of transforming growth factor β signaling and profibrotic responses in normal skin fibroblasts by peroxisome proliferator-activated receptor γ. Arthritis Rheum. 50, 1305–1318 (2004).
Nakerakanti, S. & Trojanowska, M. The role of TGF-β receptors in fibrosis. Open Rheumatol. J. 6, 156–162 (2012).
Bhattacharyya, S., Fang, F., Tourtellotte, W. & Varga, J. Egr-1: new conductor for the tissue repair orchestra directs harmony (regeneration) or cacophony (fibrosis). J. Pathol. 229, 286–297 (2013).
Leask, A. Integrin β1: a mechanosignaling sensor essential for connective tissue deposition by fibroblasts. Adv. Wound Care 2, 160–166 (2013).
Nakerakanti, S. S., Bujor, A. M. & Trojanowska, M. CCN2 is required for the TGF-β induced activation of Smad1-Erk1/2 signaling network. PLoS ONE 6, e21911 (2011).
Janmey, P. A., Wells, R. G., Assoian, R. K. & McCulloch, C. A. From tissue mechanics to transcription factors. Differentiation. 86, 112–120 (2013).
Duncan, M. R. & Berman, B. Stimulation of collagen and glycosaminoglycan production in cultured human adult dermal fibroblasts by recombinant human interleukin 6. J. Invest. Dermatol. 97, 686–692 (1991).
Jinnin, M., Ihn, H., Yamane, K. & Tamaki, K. Interleukin-13 stimulates the transcription of the human α2(I) collagen gene in human dermal fibroblasts. J. Biol. Chem. 279, 41783–41791 (2004).
Dieudé, P. et al. STAT4 is a genetic risk factor for systemic sclerosis having additive effects with IRF5on disease susceptibility and related pulmonary fibrosis. Arthritis Rheum. 60, 2472–2479 (2009).
Radstake, T. R. D. J. et al. Genome-wide association study of systemic sclerosis identifies CD247 as a new susceptibility locus. Nature Genet. 42, 426–429 (2010). This is the first GWAS reporting risk alleles for systemic sclerosis.
Dieudé, P. et al. BANK1 is a genetic risk factor for diffuse cutaneous systemic sclerosis and has additive effects with IRF5 and STAT4. Arthritis Rheum. 60, 3447–3454 (2009).
Allanore, Y. et al. Genome-wide scan identifies TNIP1, PSORS1C1, and RHOB as novel risk loci for systemic sclerosis. PLoS Genet. 7, e1002091 (2011).
Koumakis, E. et al. Brief report: candidate gene study in systemic sclerosis identifies a rare and functional variant of the TNFAIP3 locus as a risk factor for polyautoimmunity. Arthritis Rheum. 64, 2746–2752 (2012).
Diaz-Gallo, L. M. et al. Analysis of the influence of PTPN22 gene polymorphisms in systemic sclerosis. Ann. Rheum. Dis. 70, 454–462 (2011).
López-Isac, E. et al. A genome-wide association study follow-up suggests a possible role for PPARG in systemic sclerosis susceptibility. Arthritis Res. Ther. 16, R6 (2014).
Carmona, F. D. et al. New insight on the Xq28 association with systemic sclerosis. Ann. Rheum. Dis. 72, 2032–2038 (2013).
Dieudé, P. et al. Evidence of the contribution of the X chromosome to systemic sclerosis susceptibility: association with the functional IRAK1 196Phe/532Ser haplotype. Arthritis Rheum. 63, 3979–3987 (2011).
Acknowledgements
R.S. is supported in part by a grant from the US National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), 1P50AR060780‒01. O.D. is supported by funding from the European League Against Rheumatism orphan disease programme, Swiss National Science Foundation Sinergia, European Union FP‒7 DeSScipher and Rare Disease Initiative Zurich (RADIZ). M.T. is supported by grants NIAMS RO1 AR042334 and P50 AR060780. J.V. is supported by grants NIAMS AR042309 and AR064925.
Author information
Authors and Affiliations
Contributions
Introduction (J.V.); Epidemiology (Y.A.); Mechanisms/pathophysiology (J.V., M.T.); Diagnosis, screening and prevention (O.D.); Management (R.S.); Quality of life (J.P.); Outlook (C.P.D.); overview of Primer (J.V.).
Corresponding author
Ethics declarations
Competing interests
Y.A. has/had consultancy relationships and/or has received research funding in the area of systemic sclerosis and related conditions from Actelion, Bayer, Biogen, Bristol–Meyers Squibb, Inventiva, Medac, Pfizer, Roche/Genentech, Sanofi-Aventis and Servier. R.S. has/had consultancy relationships and/or has received research funding in the area of systemic sclerosis and related conditions from Actelion, Gilead, Hoffman–La Roche, Intermune, MedImmune, Novartis, Regeneron and United Therapeutics. O.D. has/had consultancy relationships and/or has received research funding in the area of systemic sclerosis and related conditions from 4D Science, Actelion, Active Biotec, Bayer, Biogen, Biovitrium, Bristol–Meyers Squibb, Boehringer, EpiPharm, Ergonex, GlaxoSmithKline, Inventiva, Medac, Novartis, Pfizer, Pharmacyclics, Roche/Genentech, Sanofi-Aventis, Serodapharm, Sinoxa and United BioSource. J.P. has/had consultancy relationships and/or has received research funding in the area of systemic sclerosis and related conditions from Actelion, Bayer, Biogen, and Roche/Genentech. C.P.D. has/had consultancy relationships and/or has received research funding in the area of systemic sclerosis and related conditions from Actelion, Biogen, Biovitrum, Boehringer Ingelheim, CSL Behring, GlaxoSmithKline, Inventiva, Novartis, Pfizer, Roche/Genetech and Sanofi-Aventis. J.V. has acted as a consultant or received research funding from Biogen/Idec, Takeda, the US National Institutes of Health, US Department of Defense. M.T. declares no competing interests.
Rights and permissions
About this article
Cite this article
Allanore, Y., Simms, R., Distler, O. et al. Systemic sclerosis. Nat Rev Dis Primers 1, 15002 (2015). https://doi.org/10.1038/nrdp.2015.2
Published:
DOI: https://doi.org/10.1038/nrdp.2015.2
This article is cited by
-
Nephrotic syndrome due to focal segmental glomerulosclerosis complicating scleroderma: a case report
Journal of Medical Case Reports (2024)
-
Validity and reliability of measurement of peripheral oxygen saturation during the 6-Minute Walk Test in patients with systemic sclerosis
Rheumatology International (2024)
-
Performance of myotonometer in the assessment of skin involvement in systemic sclerosis
Clinical Rheumatology (2024)
-
Immune profiling analysis of double-negative T cells in patients with systemic sclerosis
Clinical Rheumatology (2024)
-
Measurement equivalence of the English and French versions of the self-efficacy to manage chronic disease scale: a Scleroderma Patient-Centered Intervention Network (SPIN) study
Quality of Life Research (2024)