Key Points
-
Interferons (IFNs) are widely expressed cytokines with strong antiviral properties. There are three main types of IFNs: type I IFNs, type II IFN and type III IFNs. In addition to their role in response to viruses, type I IFNs (mainly IFNα and IFNβ) are induced by bacterial infections.
-
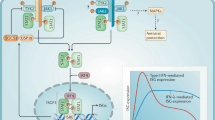
The production of type I IFNs in response to bacterial ligands is mediated through Toll-like receptor (TLR)-dependent and -independent mechanisms. TLR3, TLR4, TLR7 and TLR9 have been linked to the production of type I IFNs. The TLR-independent pathways involve the cytoplasmic nucleic acid sensors retinoic-acid-inducible gene I (RIG-I), melanoma differentiation-associated gene 5 (MDA5), stimulator of interferon genes (STING) and DNA-dependent activator of IRFs (DAI).
-
Type I IFNs activate several Janus kinase (JAK)–signal transducer and activator of transcription (STAT) signalling pathways, which regulate the transcription of target genes. In addition to the classical JAK–STAT signalling pathways, type I IFNs activate other signalling cascades.
-
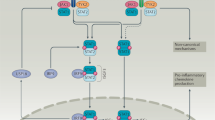
Type I IFNs mediate the regulatory functions of dendritic cells in the gut and the protective effect of TLR ligands in colonic injury.
-
Activation of type I IFN signalling has an important role in T helper cell differentiation and in the suppressive function of regulatory T cells.
-
Type I IFNs both negatively and positively regulate the activation of different types of inflammasome complex and the production of interleukin-1β.
-
In addition to their role as antiviral cytokines, type I IFNs have a wide range of immunomodulatory effects in response to bacterial infections.
-
Type I IFNs are implicated in different autoimmune and inflammatory conditions, although their role in each condition varies. Some autoimmune diseases are improved by the biological effects of type I IFNs, whereas others benefit from type I IFN inhibition.
Abstract
Interferon-α (IFNα) and IFNβ, collectively known as type I IFNs, are the major effector cytokines of the host immune response against viral infections. However, the production of type I IFNs is also induced in response to bacterial ligands of innate immune receptors and/or bacterial infections, indicating a broader physiological role for these cytokines in host defence and homeostasis than was originally assumed. The main focus of this Review is the underappreciated immunomodulatory functions of type I IFNs in health and disease. We discuss their function in the regulation of innate and adaptive immune responses, the response to bacterial ligands, inflammasome activation, intestinal homeostasis and inflammatory and autoimmune diseases.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Jenner, E. Letter to the Editor. Med. Phys. J. 12, 97–102 (1804).
Salaman, R. N. Protective inoculation against a plant virus. Nature 131, 468 (1933).
White, P. B. Lysogenic strains of V. cholerae and the influence of lysozyme on cholera phage activity. J. Pathol. Bacteriol. 44, 276–278 (1937).
Hoskins, M. A protective action of neurotropic against viscerotropic yellow fever virus in Macacus rhesus. Am. J. Trop. Med. 15, 675–680 (1935).
Isaacs, A. & Lindenmann, J. Virus interference. I. The interferon. Proc. R. Soc. Lond. B Biol. Sci. 147, 258–267 (1957).
Pestka, S., Krause, C. D. & Walter, M. R. Interferons, interferon-like cytokines, and their receptors. Immunol. Rev. 202, 8–32 (2004).
Gad, H. H. et al. Interferon-λ is functionally an interferon but structurally related to the interleukin-10 family. J. Biol. Chem. 284, 20869–20875 (2009).
Hacker, H. et al. Specificity in Toll-like receptor signalling through distinct effector functions of TRAF3 and TRAF6. Nature 439, 204–207 (2006). This study showed that TRAF3 is essential for both type I IFN and IL-10 production, but dispensable for the production of pro-inflammatory cytokines.
Schafer, S. L., Lin, R., Moore, P. A., Hiscott, J. & Pitha, P. M. Regulation of type I interferon gene expression by interferon regulatory factor-3. J. Biol. Chem. 273, 2714–2720 (1998).
Monroe, K. M., McWhirter, S. M. & Vance, R. E. Induction of type I interferons by bacteria. Cell. Microbiol. 12, 881–890 (2010).
Trinchieri, G. Type I interferon: friend or foe? J. Exp. Med. 207, 2053–2063 (2010).
Akira, S. Toll-like receptors and innate immunity. Adv. Immunol. 78, 1–56 (2001).
Barber, G. N. Innate immune DNA sensing pathways: STING, AIMII and the regulation of interferon production and inflammatory responses. Curr. Opin. Immunol. 23, 10–20 (2011).
Kawai, T. & Akira, S. The roles of TLRs, RLRs and NLRs in pathogen recognition. Int. Immunol. 21, 317–337 (2009).
Ishikawa, H., Ma, Z. & Barber, G. N. STING regulates intracellular DNA-mediated, type I interferon-dependent innate immunity. Nature 461, 788–792 (2009). A description of STING as an important mediator in the host defence response against DNA-containing pathogens and in the adjuvant activity of DNA-based vaccines.
Takaoka, A. et al. DAI (DLM-1/ZBP1) is a cytosolic DNA sensor and an activator of innate immune response. Nature 448, 501–505 (2007). The first identification of DAI as a new cytosolic DNA sensor that enhances the induction of type I IFN genes and other genes involved in innate immunity.
Kaiser, W. J., Upton, J. W. & Mocarski, E. S. Receptor-interacting protein homotypic interaction motif-dependent control of NF-κB activation via the DNA-dependent activator of IFN regulatory factors. J. Immunol. 181, 6427–6434 (2008).
Ishii, K. J. et al. TANK-binding kinase-1 delineates innate and adaptive immune responses to DNA vaccines. Nature 451, 725–729 (2008).
Chiu, Y. H., Macmillan, J. B. & Chen, Z. J. RNA polymerase III detects cytosolic DNA and induces type I interferons through the RIG-I pathway. Cell 138, 576–591 (2009).
Honda, K. & Taniguchi, T. IRFs: master regulators of signalling by Toll-like receptors and cytosolic pattern-recognition receptors. Nature Rev. Immunol. 6, 644–658 (2006).
Platanias, L. C. Mechanisms of type-I- and type-II-interferon-mediated signalling. Nature Rev. Immunol. 5, 375–386 (2005). An excellent review of the signalling mechanisms of type I IFNs, including the classical JAK–STAT pathways, as well as the MAPK and mTOR pathways.
Fasler-Kan, E., Pansky, A., Wiederkehr, M., Battegay, M. & Heim, M. H. Interferon-α activates signal transducers and activators of transcription 5 and 6 in Daudi cells. Eur. J. Biochem. 254, 514–519 (1998).
Matikainen, S. et al. Interferon-α activates multiple STAT proteins and upregulates proliferation-associated IL-2R α, c-myc, and pim-1 genes in human T cells. Blood 93, 1980–1991 (1999).
Aaronson, D. S. & Horvath, C. M. A road map for those who don't know JAK–STAT. Science 296, 1653–1655 (2002).
Platanias, L. C. & Fish, E. N. Signaling pathways activated by interferons. Exp. Hematol. 27, 1583–1592 (1999).
Sato, T., Selleri, C., Young, N. S. & Maciejewski, J. P. Inhibition of interferon regulatory factor-1 expression results in predominance of cell growth stimulatory effects of interferon-γ due to phosphorylation of Stat1 and Stat3. Blood 90, 4749–4758 (1997).
Heinrich, P. C., Behrmann, I., Muller-Newen, G., Schaper, F. & Graeve, L. Interleukin-6-type cytokine signalling through the gp130/Jak/STAT pathway. Biochem. J. 334, 297–314 (1998).
Baker, S. J., Rane, S. G. & Reddy, E. P. Hematopoietic cytokine receptor signaling. Oncogene 26, 6724–6737 (2007).
Regis, G., Pensa, S., Boselli, D., Novelli, F. & Poli, V. Ups and downs: the STAT1:STAT3 seesaw of interferon and gp130 receptor signalling. Semin. Cell Dev. Biol. 19, 351–359 (2008).
Stephanou, A., Brar, B. K., Knight, R. A. & Latchman, D. S. Opposing actions of STAT-1 and STAT-3 on the Bcl-2 and Bcl-x promoters. Cell Death Differ. 7, 329–330 (2000).
Azare, J. et al. Constitutively activated Stat3 induces tumorigenesis and enhances cell motility of prostate epithelial cells through integrin β6. Mol. Cell. Biol. 27, 4444–4453 (2007).
Dechow, T. N. et al. Requirement of matrix metalloproteinase-9 for the transformation of human mammary epithelial cells by Stat3-C. Proc. Natl Acad. Sci. USA 101, 10602–10607 (2004).
Dustin, M. L., Singer, K. H., Tuck, D. T. & Springer, T. A. Adhesion of T lymphoblasts to epidermal keratinocytes is regulated by interferon γ and is mediated by intercellular adhesion molecule 1 (ICAM-1). J. Exp. Med. 167, 1323–1340 (1988).
Yoshimura, A. Signal transduction of inflammatory cytokines and tumor development. Cancer Sci. 97, 439–447 (2006).
Brucet, M., Marques, L., Sebastian, C., Lloberas, J. & Celada, A. Regulation of murine Tap1 and Lmp2 genes in macrophages by interferon γ is mediated by STAT1 and IRF-1. Genes Immun. 5, 26–35 (2004).
Marques, L., Brucet, M., Lloberas, J. & Celada, A. STAT1 regulates lipopolysaccharide- and TNF-α-dependent expression of transporter associated with antigen processing 1 and low molecular mass polypeptide 2 genes in macrophages by distinct mechanisms. J. Immunol. 173, 1103–1110 (2004).
Ito, S. et al. Interleukin-10 inhibits expression of both interferon α- and interferon γ-induced genes by suppressing tyrosine phosphorylation of STAT1. Blood 93, 1456–1463 (1999).
Yasukawa, H. et al. IL-6 induces an anti-inflammatory response in the absence of SOCS3 in macrophages. Nature Immunol. 4, 551–556 (2003).
El Kasmi, K. C. et al. General nature of the STAT3-activated anti-inflammatory response. J. Immunol. 177, 7880–7888 (2006).
Tanabe, Y. et al. Cutting edge: role of STAT1, STAT3, and STAT5 in IFN-αβ responses in T lymphocytes. J. Immunol. 174, 609–613 (2005).
Qing, Y. & Stark, G. R. Alternative activation of STAT1 and STAT3 in response to interferon-γ. J. Biol. Chem. 279, 41679–41685 (2004).
Chang, E. Y., Guo, B., Doyle, S. E. & Cheng, G. Cutting edge: involvement of the type I IFN production and signaling pathway in lipopolysaccharide-induced IL-10 production. J. Immunol. 178, 6705–6709 (2007).
Wang, X., Chen, M., Wandinger, K. P., Williams, G. & Dhib-Jalbut, S. IFN-β-1b inhibits IL-12 production in peripheral blood mononuclear cells in an IL-10-dependent mechanism: relevance to IFN-β-1b therapeutic effects in multiple sclerosis. J. Immunol. 165, 548–557 (2000).
Ziegler-Heitbrock, L. et al. IFN-α induces the human IL-10 gene by recruiting both IFN regulatory factor 1 and Stat3. J. Immunol. 171, 285–290 (2003).
Wang, H. et al. The role of glycogen synthase kinase 3 in regulating IFN-β-mediated IL-10 production. J. Immunol. 186, 675–684 (2011).
Uddin, S. et al. Activation of the p38 mitogen-activated protein kinase by type I interferons. J. Biol. Chem. 274, 30127–30131 (1999).
Uddin, S. et al. The Rac1/p38 mitogen-activated protein kinase pathway is required for interferon α-dependent transcriptional activation but not serine phosphorylation of Stat proteins. J. Biol. Chem. 275, 27634–27640 (2000).
Li, Y. et al. Role of p38α Map kinase in type I interferon signaling. J. Biol. Chem. 279, 970–979 (2004).
Mayer, I. A. et al. The p38 MAPK pathway mediates the growth inhibitory effects of interferon-α in BCR-ABL-expressing cells. J. Biol. Chem. 276, 28570–28577 (2001).
Ishida, H. et al. Involvement of p38 signaling pathway in interferon-α-mediated antiviral activity toward hepatitis C virus. Biochem. Biophys. Res. Commun. 321, 722–727 (2004).
David, M. et al. Requirement for MAP kinase (ERK2) activity in interferon α- and interferon β-stimulated gene expression through STAT proteins. Science 269, 1721–1723 (1995).
Wang, F. et al. Disruption of Erk-dependent type I interferon induction breaks the myxoma virus species barrier. Nature Immunol. 5, 1266–1274 (2004).
Uddin, S. et al. Interferon-α engages the insulin receptor substrate-1 to associate with the phosphatidylinositol 3′-kinase. J. Biol. Chem. 270, 15938–15941 (1995).
Kaur, S. et al. Role of the Akt pathway in mRNA translation of interferon-stimulated genes. Proc. Natl Acad. Sci. USA 105, 4808–4813 (2008).
Kaur, S. et al. Dual regulatory roles of phosphatidylinositol 3-kinase in IFN signaling. J. Immunol. 181, 7316–7323 (2008).
Lekmine, F. et al. Activation of the p70 S6 kinase and phosphorylation of the 4E-BP1 repressor of mRNA translation by type I interferons. J. Biol. Chem. 278, 27772–27780 (2003).
Lekmine, F. et al. Interferon-γ engages the p70 S6 kinase to regulate phosphorylation of the 40S S6 ribosomal protein. Exp. Cell Res. 295, 173–182 (2004).
Matsumoto, A. et al. Interferon-α-induced mTOR activation is an anti-hepatitis C virus signal via the phosphatidylinositol 3-kinase–Akt-independent pathway. J. Gastroenterol. 44, 856–863 (2009).
Round, J. L. & Mazmanian, S. K. The gut microbiota shapes intestinal immune responses during health and disease. Nature Rev. Immunol. 9, 313–323 (2009).
Katakura, K. et al. Toll-like receptor 9-induced type I IFN protects mice from experimental colitis. J. Clin. Invest. 115, 695–702 (2005). This study demonstrated that the protective effect of TLR9 signalling in colonic injury is mediated by type I IFNs.
Rachmilewitz, D. et al. Toll-like receptor 9 signaling mediates the anti-inflammatory effects of probiotics in murine experimental colitis. Gastroenterology 126, 520–528 (2004).
Vijay-Kumar, M. et al. Activation of Toll-like receptor 3 protects against DSS-induced acute colitis. Inflamm. Bowel Dis. 13, 856–864 (2007).
McFarland, A. P. et al. Localized delivery of interferon-β by Lactobacillus exacerbates experimental colitis. PloS ONE 6, e16967 (2011).
Abe, K. et al. Conventional dendritic cells regulate the outcome of colonic inflammation independently of T cells. Proc. Natl Acad. Sci. USA 104, 17022–17027 (2007).
Hall, J. A. et al. Commensal DNA limits regulatory T cell conversion and is a natural adjuvant of intestinal immune responses. Immunity 29, 637–649 (2008).
Hofmann, C. et al. T cell-dependent protective effects of CpG motifs of bacterial DNA in experimental colitis are mediated by CD11c+ dendritic cells. Gut 59, 1347–1354 (2010).
Bleich, A. et al. CpG motifs of bacterial DNA exert protective effects in mouse models of IBD by antigen-independent tolerance induction. Gastroenterology 136, 278–287 (2009).
Bilsborough, J., George, T. C., Norment, A. & Viney, J. L. Mucosal CD8α+ DC, with a plasmacytoid phenotype, induce differentiation and support function of T cells with regulatory properties. Immunology 108, 481–492 (2003).
Levings, M. K. et al. IFN-α and IL-10 induce the differentiation of human type 1 T regulatory cells. J. Immunol. 166, 5530–5539 (2001).
Szabo, S. J. et al. Distinct effects of T-bet in TH1 lineage commitment and IFN-γ production in CD4 and CD8 T cells. Science 295, 338–342 (2002).
Cho, S. S. et al. Activation of STAT4 by IL-12 and IFN-α: evidence for the involvement of ligand-induced tyrosine and serine phosphorylation. J. Immunol. 157, 4781–4789 (1996).
Rogge, L. et al. The role of Stat4 in species-specific regulation of Th cell development by type I IFNs. J. Immunol. 161, 6567–6574 (1998).
Cousens, L. P. et al. Two roads diverged: interferon α/β- and interleukin 12-mediated pathways in promoting T cell interferon γ responses during viral infection. J. Exp. Med. 189, 1315–1328 (1999).
Nguyen, K. B. et al. Critical role for STAT4 activation by type 1 interferons in the interferon-γ response to viral infection. Science 297, 2063–2066 (2002).
Berenson, L. S., Farrar, J. D., Murphy, T. L. & Murphy, K. M. Frontline: absence of functional STAT4 activation despite detectable tyrosine phosphorylation induced by murine IFN-α. Eur. J. Immunol. 34, 2365–2374 (2004).
Farrar, J. D., Smith, J. D., Murphy, T. L. & Murphy, K. M. Recruitment of Stat4 to the human interferon-α/β receptor requires activated Stat2. J. Biol. Chem. 275, 2693–2697 (2000).
Berenson, L. S., Gavrieli, M., Farrar, J. D., Murphy, T. L. & Murphy, K. M. Distinct characteristics of murine STAT4 activation in response to IL-12 and IFN-α. J. Immunol. 177, 5195–5203 (2006).
Huber, J. P. & Farrar, J. D. Regulation of effector and memory T-cell functions by type I interferon. Immunology 132, 466–474 (2011).
Matikainen, S. et al. IFN-α and IL-18 synergistically enhance IFN-γ production in human NK cells: differential regulation of Stat4 activation and IFN-γ gene expression by IFN-α and IL-12. Eur. J. Immunol. 31, 2236–2245 (2001).
Strengell, M., Julkunen, I. & Matikainen, S. IFN-α regulates IL-21 and IL-21R expression in human NK and T cells. J. Leukoc. Biol. 76, 416–422 (2004).
Harrington, L. E. et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nature Immunol. 6, 1123–1132 (2005).
Moschen, A. R., Geiger, S., Krehan, I., Kaser, A. & Tilg, H. Interferon-α controls IL-17 expression in vitro and in vivo. Immunobiology 213, 779–787 (2008).
Qiu, H. et al. Type I IFNs enhance susceptibility to Chlamydia muridarum lung infection by enhancing apoptosis of local macrophages. J. Immunol. 181, 2092–2102 (2008).
Karaghiosoff, M. et al. Central role for type I interferons and Tyk2 in lipopolysaccharide-induced endotoxin shock. Nature Immunol. 4, 471–477 (2003).
Vandenbark, A. A. et al. Interferon-β-1a treatment increases CD56bright natural killer cells and CD4+CD25+ Foxp3 expression in subjects with multiple sclerosis. J. Neuroimmunol. 215, 125–128 (2009).
Zhou, X. et al. Instability of the transcription factor Foxp3 leads to the generation of pathogenic memory T cells in vivo. Nature Immunol. 10, 1000–1007 (2009).
Oldenhove, G. et al. Decrease of Foxp3+ Treg cell number and acquisition of effector cell phenotype during lethal infection. Immunity 31, 772–786 (2009).
Dominguez-Villar, M., Baecher-Allan, C. M. & Hafler, D. A. Identification of T helper type 1-like, Foxp3+ regulatory T cells in human autoimmune disease. Nature Med. 17, 673–675 (2011).
Schroder, K. & Tschopp, J. The inflammasomes. Cell 140, 821–832 (2010).
Guarda, G. et al. Type I interferon inhibits interleukin-1 production and inflammasome activation. Immunity 34, 213–223 (2011). An important study showing that type I IFN signalling inhibits NLRP1 and NLRP3 inflammasomes in a two-step process through STAT1 and IL-10.
Henry, T., Brotcke, A., Weiss, D. S., Thompson, L. J. & Monack, D. M. Type I interferon signaling is required for activation of the inflammasome during Francisella infection. J. Exp. Med. 204, 987–994 (2007).
Fernandes-Alnemri, T. et al. The AIM2 inflammasome is critical for innate immunity to Francisella tularensis. Nature Immunol. 11, 385–393 (2010).
Fujita, T. et al. Induction of the transcription factor IRF-1 and interferon-β mRNAs by cytokines and activators of second-messenger pathways. Proc. Natl Acad. Sci. USA 86, 9936–9940 (1989).
Rivieccio, M. A. et al. The cytokine IL-1β activates IFN response factor 3 in human fetal astrocytes in culture. J. Immunol. 174, 3719–3726 (2005).
Gonzalez-Navajas, J. M. et al. Interleukin 1 receptor signaling regulates DUBA expression and facilitates Toll-like receptor 9-driven antiinflammatory cytokine production. J. Exp. Med. 207, 2799–2807 (2010).
Tseng, P. H. et al. Different modes of ubiquitination of the adaptor TRAF3 selectively activate the expression of type I interferons and proinflammatory cytokines. Nature Immunol. 11, 70–75 (2010). An elegant study showing the different effects of K63- and K48-linked polyubiquitylation on the biological functions of TRAF3.
Kayagaki, N. et al. DUBA: a deubiquitinase that regulates type I interferon production. Science 318, 1628–1632 (2007). The first report that DUBA binds to TRAF3 and selectively cleaves the K63-linked polyubiquitin chains, thereby dampening type I IFN production.
Lebeis, S. L., Powell, K. R., Merlin, D., Sherman, M. A. & Kalman, D. Interleukin-1 receptor signaling protects mice from lethal intestinal damage caused by the attaching and effacing pathogen Citrobacter rodentium. Infect. Immun. 77, 604–614 (2009).
Kojouharoff, G. et al. Neutralization of tumour necrosis factor (TNF) but not of IL-1 reduces inflammation in chronic dextran sulphate sodium-induced colitis in mice. Clin. Exp. Immunol. 107, 353–358 (1997).
Auerbuch, V., Brockstedt, D. G., Meyer-Morse, N., O'Riordan, M. & Portnoy, D. A. Mice lacking the type I interferon receptor are resistant to Listeria monocytogenes. J. Exp. Med. 200, 527–533 (2004).
Carrero, J. A., Calderon, B. & Unanue, E. R. Type I interferon sensitizes lymphocytes to apoptosis and reduces resistance to Listeria infection. J. Exp. Med. 200, 535–540 (2004).
Stanley, S. A., Johndrow, J. E., Manzanillo, P. & Cox, J. S. The type I IFN response to infection with Mycobacterium tuberculosis requires ESX-1-mediated secretion and contributes to pathogenesis. J. Immunol. 178, 3143–3152 (2007).
Nagarajan, U. M. et al. Type I interferon signaling exacerbates Chlamydia muridarum genital infection in a murine model. Infect. Immun. 76, 4642–4648 (2008).
Henry, T. et al. Type I IFN signaling constrains IL-17A/F secretion by γδ T cells during bacterial infections. J. Immunol. 184, 3755–3767 (2010).
Carrero, J. A. & Unanue, E. R. Lymphocyte apoptosis as an immune subversion strategy of microbial pathogens. Trends Immunol. 27, 497–503 (2006).
Mancuso, G. et al. Type I IFN signaling is crucial for host resistance against different species of pathogenic bacteria. J. Immunol. 178, 3126–3133 (2007).
Coers, J., Vance, R. E., Fontana, M. F. & Dietrich, W. F. Restriction of Legionella pneumophila growth in macrophages requires the concerted action of cytokine and Naip5/Ipaf signalling pathways. Cell. Microbiol. 9, 2344–2357 (2007).
Burger, D. & Travis, S. Conventional medical management of inflammatory bowel disease. Gastroenterology 140, 1827–1837 (2011).
Musch, E., Andus, T., Malek, M., Chrissafidou, A. & Schulz, M. Successful treatment of steroid refractory active ulcerative colitis with natural interferon-β — an open long-term trial. Z. Gastroenterol. 45, 1235–1240 (2007).
Nikolaus, S. et al. Interferon β-1a in ulcerative colitis: a placebo controlled, randomised, dose escalating study. Gut 52, 1286–1290 (2003).
Madsen, S. M. et al. An open-labeled, randomized study comparing systemic interferon-α-2A and prednisolone enemas in the treatment of left-sided ulcerative colitis. Am. J. Gastroenterol. 96, 1807–1815 (2001).
Pena Rossi, C. et al. Interferon β-1a for the maintenance of remission in patients with Crohn's disease: results of a phase II dose-finding study. BMC Gastroenterol. 9, 22 (2009).
Pena-Rossi, C. et al. Clinical trial: a multicentre, randomized, double-blind, placebo-controlled, dose-finding, phase II study of subcutaneous interferon-β-1a in moderately active ulcerative colitis. Aliment. Pharmacol. Ther. 28, 758–767 (2008).
Gasche, C. et al. Prospective evaluation of interferon-α in treatment of chronic active Crohn's disease. Dig. Dis. Sci. 40, 800–804 (1995).
Musch, E. et al. Interferon-β-1a for the treatment of steroid-refractory ulcerative colitis: a randomized, double-blind, placebo-controlled trial. Clin. Gastroenterol. Hepatol. 3, 581–586 (2005).
Mitoro, A. et al. Exacerbation of ulcerative colitis during α-interferon therapy for chronic hepatitis C. Intern. Med. 32, 327–331 (1993).
Watanabe, T. et al. A case of exacerbation of ulcerative colitis induced by combination therapy with PEG-interferon α-2b and ribavirin. Gut 55, 1682–1683 (2006).
Schott, E. et al. Development of ulcerative colitis in a patient with multiple sclerosis following treatment with interferon β1a. World J. Gastroenterol. 13, 3638–3640 (2007).
Freeman, H. J., Chopra, A., Clandinin, M. T. & Thomson, A. B. Recent advances in celiac disease. World J. Gastroenterol. 17, 2259–2272 (2011).
MacDonald, T. T. & Spencer, J. Evidence that activated mucosal T cells play a role in the pathogenesis of enteropathy in human small intestine. J. Exp. Med. 167, 1341–1349 (1988).
Neurath, M. F., Finotto, S. & Glimcher, L. H. The role of Th1/Th2 polarization in mucosal immunity. Nature Med. 8, 567–573 (2002).
Monteleone, G., Pender, S. L., Wathen, N. C. & MacDonald, T. T. Interferon-α drives T cell-mediated immunopathology in the intestine. Eur. J. Immunol. 31, 2247–2255 (2001).
Di Sabatino, A. et al. Evidence for the role of interferon-alfa production by dendritic cells in the Th1 response in celiac disease. Gastroenterology 133, 1175–1187 (2007).
Molberg, O. et al. Tissue transglutaminase selectively modifies gliadin peptides that are recognized by gut-derived T cells in celiac disease. Nature Med. 4, 713–717 (1998).
Molberg, O. et al. Gliadin specific, HLA DQ2-restricted T cells are commonly found in small intestinal biopsies from coeliac disease patients, but not from controls. Scand. J. Immunol. 46, 103–109 (1997).
Kagnoff, M. F. Celiac disease: pathogenesis of a model immunogenetic disease. J. Clin. Invest. 117, 41–49 (2007).
Ting, J. P. & Trowsdale, J. Genetic control of MHC class II expression. Cell 109, S21–S33 (2002).
Cammarota, G., Cuoco, L., Cianci, R., Pandolfi, F. & Gasbarrini, G. Onset of coeliac disease during treatment with interferon for chronic hepatitis C. Lancet 356, 1494–1495 (2000).
Zheng, Y. et al. Interleukin-22, a TH17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature 445, 648–651 (2007).
Nestle, F. O. et al. Plasmacytoid predendritic cells initiate psoriasis through interferon-α production. J. Exp. Med. 202, 135–143 (2005).
Dzionek, A. et al. BDCA-2, a novel plasmacytoid dendritic cell-specific type II C-type lectin, mediates antigen capture and is a potent inhibitor of interferon α/β induction. J. Exp. Med. 194, 1823–1834 (2001).
Hua, J., Kirou, K., Lee, C. & Crow, M. K. Functional assay of type I interferon in systemic lupus erythematosus plasma and association with anti-RNA binding protein autoantibodies. Arthritis Rheum. 54, 1906–1916 (2006).
Kirou, K. A. et al. Activation of the interferon-α pathway identifies a subgroup of systemic lupus erythematosus patients with distinct serologic features and active disease. Arthritis Rheum. 52, 1491–1503 (2005).
Blanco, P., Palucka, A. K., Gill, M., Pascual, V. & Banchereau, J. Induction of dendritic cell differentiation by IFN-α in systemic lupus erythematosus. Science 294, 1540–1543 (2001).
Jego, G. et al. Plasmacytoid dendritic cells induce plasma cell differentiation through type I interferon and interleukin 6. Immunity 19, 225–234 (2003).
Yao, Y. et al. Neutralization of interferon-α/β-inducible genes and downstream effect in a phase I trial of an anti-interferon-α monoclonal antibody in systemic lupus erythematosus. Arthritis Rheum. 60, 1785–1796 (2009).
Ann Marrie, R. & Rudick, R. A. Drug insight: interferon treatment in multiple sclerosis. Nature Clin. Pract. Neurol. 2, 34–44 (2006).
Bendtzen, K. Critical review: assessment of interferon-β immunogenicity in multiple sclerosis. J. Interferon Cytokine Res. 30, 759–766 (2010).
The IFNB Multiple Sclerosis Study Group. Interferon β-1b is effective in relapsing-remitting multiple sclerosis. I. Clinical results of a multicenter, randomized, double-blind, placebo-controlled trial. Neurology 43, 655–661 (1993).
Teige, I. et al. IFN-β gene deletion leads to augmented and chronic demyelinating experimental autoimmune encephalomyelitis. J. Immunol. 170, 4776–4784 (2003).
Brod, S. A. & Burns, D. K. Suppression of relapsing experimental autoimmune encephalomyelitis in the SJL/J mouse by oral administration of type I interferons. Neurology 44, 1144–1148 (1994).
Prinz, M. et al. Distinct and nonredundant in vivo functions of IFNAR on myeloid cells limit autoimmunity in the central nervous system. Immunity 28, 675–686 (2008).
Guo, B., Chang, E. Y. & Cheng, G. The type I IFN induction pathway constrains Th17-mediated autoimmune inflammation in mice. J. Clin. Invest. 118, 1680–1690 (2008).
Teige, I., Liu, Y. & Issazadeh-Navikas, S. IFN-β inhibits T cell activation capacity of central nervous system APCs. J. Immunol. 177, 3542–3553 (2006).
Rudick, R. A. et al. Interferon β induces interleukin-10 expression: relevance to multiple sclerosis. Ann. Neurol. 40, 618–627 (1996).
Ferrantini, M., Capone, I. & Belardelli, F. Interferon-α and cancer: mechanisms of action and new perspectives of clinical use. Biochimie 89, 884–893 (2007).
Rizza, P., Moretti, F. & Belardelli, F. Recent advances on the immunomodulatory effects of IFN-α: implications for cancer immunotherapy and autoimmunity. Autoimmunity 43, 204–209 (2010).
Ferrantini, M. & Belardelli, F. Gene therapy of cancer with interferon: lessons from tumor models and perspectives for clinical applications. Semin. Cancer Biol. 10, 145–157 (2000).
Di Pucchio, T. et al. Immunization of stage IV melanoma patients with Melan-A/MART-1 and gp100 peptides plus IFN-α results in the activation of specific CD8+ T cells and monocyte/dendritic cell precursors. Cancer Res. 66, 4943–4951 (2006).
Lapenta, C. et al. IFN-α-conditioned dendritic cells are highly efficient in inducing cross-priming CD8+ T cells against exogenous viral antigens. Eur. J. Immunol. 36, 2046–2060 (2006).
Lapenta, C. et al. Potent immune response against HIV-1 and protection from virus challenge in hu-PBL-SCID mice immunized with inactivated virus-pulsed dendritic cells generated in the presence of IFN-α. J. Exp. Med. 198, 361–367 (2003).
Santodonato, L. et al. Monocyte-derived dendritic cells generated after a short-term culture with IFN-α and granulocyte-macrophage colony-stimulating factor stimulate a potent Epstein-Barr virus-specific CD8+ T cell response. J. Immunol. 170, 5195–5202 (2003).
Acknowledgements
We thank S. Herdman for his editorial assistance. This work was supported by US National Institutes of Health grants AI068685, AI077989, AI095623 and DK35108; grants from the Crohn's and Colitis Foundation of America; and grant CP10/00417 from Instituto de Salud Carlos III, Madrid, Spain.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- Viral interference
-
The antagonistic or inhibitory effect induced by one virus or its components on the propagation of another virus.
- IFN-stimulated genes
-
(ISGs). These genes contain promoters that are responsive to interferon (IFN) signalling, and they are responsible for the antiviral and immunomodulatory properties of IFNs. Over 400 such genes have been identified by microarray analyses. Some, such as RNA-activated protein kinase, ribonuclease L, MX1 (myxovirus resistance 1) and ISG15 (IFN-stimulated gene of 15 kDa), have well-documented antiviral activities, but the precise biological function of most of these genes is unknown.
- Inflammasome
-
A cytosolic multiprotein complex that activates caspase 1 and regulates the release of IL-1β and IL-18 in response to exogenous pathogens and endogenous danger signals. This complex minimally consists of a danger-sensing initiator component and the effector component, which is mature caspase 1.
- Coeliac disease
-
An immune-mediated enteropathy triggered by intolerance to dietary ingestion of glutamine- and proline-rich proteins, collectively known as gluten, which is present in wheat, barley, rye and other grains. This disease results in gastrointestinal symptoms such as diarrhoea, nutrient malabsorption and weight loss.
- Multiple sclerosis
-
A chronic inflammatory disease of the central nervous system that causes the progressive destruction of the myelin sheaths around axons in any area of the brain, optic nerve and spinal cord. This results in slower nerve impulses.
- Peyer's patches
-
Collections of lymphoid tissue that are located in the mucosa of the small intestine, with an outer epithelial layer that consists of specialized epithelial cells called M cells.
- Systemic lupus erythematosus
-
(SLE). An autoimmune disease characterized by the presence of circulating immune complexes that contain antinuclear antibodies bound to self nucleic acids and other nuclear antigens.
- Inflammatory bowel disease
-
(IBD). A chronic inflammatory condition that affects the intestinal tract. The proposed pathogenesis of IBD involves a complex model that includes abnormalities of innate immune function and their relationship with the commensal microbiota, inappropriate release of pro-inflammatory cytokines and other mediators, alterations of the intestinal epithelial barrier, and a cytokine imbalance that promotes the pro-inflammatory activity of adaptive immune cells.
Rights and permissions
About this article
Cite this article
González-Navajas, J., Lee, J., David, M. et al. Immunomodulatory functions of type I interferons. Nat Rev Immunol 12, 125–135 (2012). https://doi.org/10.1038/nri3133
Published:
Issue Date:
DOI: https://doi.org/10.1038/nri3133
This article is cited by
-
A pilot study of the differentiated landscape of peripheral blood mononuclear cells from children with incomplete versus complete Kawasaki disease
World Journal of Pediatrics (2024)
-
Pretreatment of UC-MSCs with IFN-α2 improves treatment of liver fibrosis by recruiting neutrophils
Journal of Translational Medicine (2023)
-
Single cell transcriptomics shows that malaria promotes unique regulatory responses across multiple immune cell subsets
Nature Communications (2023)
-
Corticosteroids reduce pathologic interferon responses by downregulating STAT1 in patients with high-risk COVID-19
Experimental & Molecular Medicine (2023)
-
Emerging principles of cytokine pharmacology and therapeutics
Nature Reviews Drug Discovery (2023)