Abstract
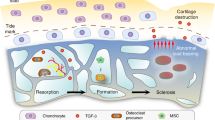
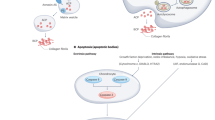
Osteoarthritis (OA) refers to a group of mechanically-induced joint disorders to which both genetic and acquired factors contribute. Current pathophysiological concepts focus on OA as a disease of the whole joint. Within these models, the functional unit formed by the articular cartilage and the subchondral bone seems to be of particular interest. Cartilage and bone receive and dissipate the stress associated with movement and loading, and are therefore continuously challenged biomechanically. Recent data support the view that cartilage and bone can communicate over the calcified tissue barrier; vessels reach out from bone into the cartilage zone, patches of uncalcified cartilage are in contact with bone, and microcracks and fissures further facilitate transfer of molecules. Several molecular signaling pathways such as bone morphogenetic proteins and Wnts are hypothesized to have a role in OA and can activate cellular and molecular processes in both cartilage and bone cells. In addition, intracellular activation of different kinase cascades seems to be involved in the molecular crosstalk between cartilage and bone cells. Further research is required to integrate these different elements into a comprehensive approach that will increase our understanding of the disease processes in OA, and that could lead to the development of specific therapeutics or treatment strategies.
Key Points
-
Osteoarthritis (OA) is a disease of the whole joint, to which changes in cartilage, bone, bone marrow, synovium, menisci, ligaments and neural tissue contribute
-
Subchondral bone changes, with increased metabolism and sclerosis, are often the first detectable alterations in the OA process
-
Increased subchondral remodeling might lead to decreased local mineralization
-
Molecular crosstalk between the cartilage and bone is possible, and it increases with progression of OA
-
Identifying risk factors for disease and halting disease progression are the main clinical challenges in OA
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Hunter, D. J. & Felson, D. T. Osteoarthritis. BMJ 332, 639–642 (2006).
Lane, N. E. Clinical practice. Osteoarthritis of the hip. N. Engl. J. Med. 357, 1413–1421 (2007).
Felson, D. T. Clinical practice. Osteoarthritis of the knee. N. Engl. J. Med. 354, 841–848 (2006).
Lawrence, R. C. et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 58, 26–35 (2008).
Berenbaum, F. New horizons and perspectives in the treatment of osteoarthritis. Arthritis Res. Ther. 10 (Suppl. 2), 1 (2008).
Felson, D. T., Anderson, J. J. & Meenan, R. F. The comparative efficacy and toxicity of second-line drugs in rheumatoid arthritis. Results of two metaanalyses. Arthritis Rheum. 33, 1449–1461 (1990).
Li, Y., Xu, L. & Olsen, B. R. Lessons from genetic forms of osteoarthritis for the pathogenesis of the disease. Osteoarthritis Cartilage 15, 1101–1105 (2007).
Miyamoto, Y. et al. A functional polymorphism in the 5′ UTR of GDF5 is associated with susceptibility to osteoarthritis. Nat. Genet. 39, 529–533 (2007).
Valdes, A. M. & Spector, T. D. The contribution of genes to osteoarthritis. Rheum. Dis. Clin. North Am. 34, 581–603 (2008).
Valdes, A. M. & Spector, T. D. The clinical relevance of genetic susceptibility to osteoarthritis. Best Pract. Res. Clin. Rheumatol. 24, 3–14 (2010).
Southam, L. et al. An SNP in the 5′-UTR of GDF5 is associated with osteoarthritis susceptibility in Europeans and with in vivo differences in allelic expression in articular cartilage. Hum. Mol. Genet. 16, 2226–2232 (2007).
Evangelou, E. et al. Large-scale analysis of association between GDF5 and FRZB variants and osteoarthritis of the hip, knee, and hand. Arthritis Rheum. 60, 1710–1721 (2009).
Baker-LePain, J. C. & Lane, N. E. Relationship between joint shape and the development of osteoarthritis. Curr. Opin. Rheumatol. 22, 538–543 (2010).
Brandt, K. D., Radin, E. L., Dieppe, P. A. & van de Putte, L. Yet more evidence that osteoarthritis is not a cartilage disease. Ann. Rheum. Dis. 65, 1261–1264 (2006).
Sellam, J. & Berenbaum, F. The role of synovitis in pathophysiology and clinical symptoms of osteoarthritis. Nat. Rev. Rheumatol. 6, 625–635 (2010).
Luyten, F. P., Lories, R. J., Verschueren, P., de Vlam, K. & Westhovens, R. Contemporary concepts of inflammation, damage and repair in rheumatic diseases. Best Pract. Res. Clin. Rheumatol. 20, 829–848 (2006).
Goldring, M. B. & Goldring, S. R. Articular cartilage and subchondral bone in the pathogenesis of osteoarthritis. Ann. NY Acad. Sci. 1192, 230–237 (2010).
Thambyah, A. & Broom, N. On new bone formation in the pre-osteoarthritic joint. Osteoarthritis Cartilage 17, 456–463 (2009).
Radin, E. L. & Rose, R. M. Role of subchondral bone in the initiation and progression of cartilage damage. Clin. Orthop. Relat. Res. 213, 34–40 (1986).
Day, J. S. et al. A decreased subchondral trabecular bone tissue elastic modulus is associated with pre-arthritic cartilage damage. J. Orthop. Res. 19, 914–918 (2001).
Brown, T. D., Radin, E. L., Martin, R. B. & Burr, D. B. Finite element studies of some juxtarticular stress changes due to localized subchondral stiffening. J. Biomech. 17, 11–24 (1984).
Blair-Levy, J. M. et al. A type I collagen defect leads to rapidly progressive osteoarthritis in a mouse model. Arthritis Rheum. 58, 1096–1106 (2008).
Muraoka, T., Hagino, H., Okano, T., Enokida, M. & Teshima, R. Role of subchondral bone in osteoarthritis development: a comparative study of two strains of guinea pigs with and without spontaneously occurring osteoarthritis. Arthritis Rheum. 56, 3366–3374 (2007).
Daans, M., Luyten, F. P. & Lories, R. J. GDF5 deficiency in mice is associated with instability-driven joint damage, gait and subchondral bone changes. Ann. Rheum. Dis. doi:10.1136/ard.2010.134619
Roemer, F. W. et al. Change in MRI-detected subchondral bone marrow lesions is associated with cartilage loss: the MOST Study. A longitudinal multicentre study of knee osteoarthritis. Ann. Rheum. Dis. 68, 1461–1465 (2009).
Javaid, M. K. et al. Pre-radiographic MRI findings are associated with onset of knee symptoms: the MOST study. Osteoarthritis Cartilage 18, 323–328 (2010).
Raynauld, J. P. et al. Correlation between bone lesion changes and cartilage volume loss in patients with osteoarthritis of the knee as assessed by quantitative magnetic resonance imaging over a 24-month period. Ann. Rheum. Dis. 67, 683–688 (2008).
Neogi, T. et al. Cartilage loss occurs in the same subregions as subchondral bone attrition: a within-knee subregion-matched approach from the Multicenter Osteoarthritis Study. Arthritis Rheum. 61, 1539–1544 (2009).
Roemer, F. W. et al. Subchondral bone marrow lesions are highly associated with, and predict subchondral bone attrition longitudinally: the MOST study. Osteoarthritis Cartilage 18, 47–53 (2010).
Neogi, T. et al. Subchondral bone attrition may be a reflection of compartment-specific mechanical load: the MOST Study. Ann. Rheum. Dis. 69, 841–844 (2010).
Berry, J. L., Thaeler-Oberdoerster, D. A. & Greenwald, A. S. Subchondral pathways to the superior surface of the human talus. Foot Ankle 7, 2–9 (1986).
Imhof, H., Breitenseher, M., Kainberger, F., Rand, T. & Trattnig, S. Importance of subchondral bone to articular cartilage in health and disease. Top. Magn. Reson. Imaging 10, 180–192 (1999).
Lyons, T. J., McClure, S. F., Stoddart, R. W. & McClure, J. The normal human chondro-osseous junctional region: evidence for contact of uncalcified cartilage with subchondral bone and marrow spaces. BMC Musculoskelet. Disord. 7, 52 (2006).
Walsh, D. A. et al. Angiogenesis and nerve growth factor at the osteochondral junction in rheumatoid arthritis and osteoarthritis. Rheumatology (Oxford) 49, 1852–1861 (2010).
Madry, H., van Dijk, C. N. & Mueller-Gerbl, M. The basic science of the subchondral bone. Knee Surg. Sports Traumatol. Arthrosc. 18, 419–433 (2010).
Bashir, A., Gray, M. L., Boutin, R. D. & Burstein, D. Glycosaminoglycan in articular cartilage: in vivo assessment with delayed Gd(DTPA)(2-)-enhanced MR imaging. Radiology 205, 551–558 (1997).
Pan, J. et al. In situ measurement of transport between subchondral bone and articular cartilage. J. Orthop. Res. 27, 1347–1352 (2009).
Hwang, J. et al. Increased hydraulic conductance of human articular cartilage and subchondral bone plate with progression of osteoarthritis. Arthritis Rheum. 58, 3831–3842 (2008).
Luyten, F. P., Tylzanowski, P. & Lories, R. J. Wnt signaling and osteoarthritis. Bone 44, 522–527 (2009).
van der Kraan, P. M., Davidson, E. N. & van den Berg, W. B. Bone morphogenetic proteins and articular cartilage: to serve and protect or a wolf in sheep's clothing? Osteoarthritis Cartilage 18, 735–741 (2010).
Blom, A. B., van Lent, P. L., van der Kraan, P. M. & van den Berg, W. B. To seek shelter from the WNT in osteoarthritis? WNT-signaling as a target for osteoarthritis therapy. Curr. Drug Targets 11, 620–629 (2010).
Zhu, M. et al. Activation of β-catenin signaling in articular chondrocytes leads to osteoarthritis-like phenotype in adult β-catenin conditional activation mice. J. Bone Miner. Res. 24, 12–21 (2009).
Zhu, M. et al. Inhibition of β-catenin signaling in articular chondrocytes results in articular cartilage destruction. Arthritis Rheum. 58, 2053–2064 (2008).
Lories, R. J. et al. Articular cartilage and biomechanical properties of the long bones in Frzb-knockout mice. Arthritis Rheum. 56, 4095–4103 (2007).
Bodine, P. V. et al. The Wnt antagonist secreted frizzled-related protein-1 is a negative regulator of trabecular bone formation in adult mice. Mol. Endocrinol. 18, 1222–1237 (2004).
Weng, L. H., Wang, C. J., Ko, J. Y., Sun, Y. C. & Wang, F. S. Control of Dkk-1 ameliorates chondrocyte apoptosis, cartilage destruction, and subchondral bone deterioration in osteoarthritic knees. Arthritis Rheum. 62, 1393–1402 (2010).
Lories, R. J. & Luyten, F. P. Bone morphogenetic protein signaling and arthritis. Cytokine Growth Factor Rev. 20, 467–473 (2009).
Westacott, C. I., Webb, G. R., Warnock, M. G., Sims, J. V. & Elson, C. J. Alteration of cartilage metabolism by cells from osteoarthritic bone. Arthritis Rheum. 40, 1282–1291 (1997).
Hilal, G., Martel-Pelletier, J., Pelletier, J. P., Ranger, P. & Lajeunesse, D. Osteoblast-like cells from human subchondral osteoarthritic bone demonstrate an altered phenotype in vitro: possible role in subchondral bone sclerosis. Arthritis Rheum. 41, 891–899 (1998).
Massicotte, F. et al. Can altered production of interleukin-1β, interleukin-6, transforming growth factor-β and prostaglandin E2 by isolated human subchondral osteoblasts identify two subgroups of osteoarthritic patients? Osteoarthritis Cartilage 10, 491–500 (2002).
Sanchez, C. et al. Phenotypic characterization of osteoblasts from the sclerotic zones of osteoarthritic subchondral bone. Arthritis Rheum. 58, 442–455 (2008).
Mutabaruka, M. S., Aoulad Aissa, M., Delalandre, A., Lavigne, M. & Lajeunesse, D. Local leptin production in osteoarthritis subchondral osteoblasts may be responsible for their abnormal phenotypic expression. Arthritis Res. Ther. 12, R20 (2010).
Sanchez, C. et al. Osteoblasts from the sclerotic subchondral bone downregulate aggrecan but upregulate metalloproteinases expression by chondrocytes. This effect is mimicked by interleukin-6, -1β and oncostatin M pre-treated non-sclerotic osteoblasts. Osteoarthritis Cartilage 13, 979–987 (2005).
Sanchez, C. et al. Subchondral bone osteoblasts induce phenotypic changes in human osteoarthritic chondrocytes. Osteoarthritis Cartilage 13, 988–997 (2005).
Lin, Y. Y. et al. Applying an excessive mechanical stress alters the effect of subchondral osteoblasts on chondrocytes in a co-culture system. Eur. J. Oral Sci. 118, 151–158 (2010).
Prasadam, I. et al. ERK-1/2 and p38 in the regulation of hypertrophic changes of normal articular cartilage chondrocytes induced by osteoarthritic subchondral osteoblasts. Arthritis Rheum. 62, 1349–1360 (2010).
Prasadam, I. et al. Osteoarthritic cartilage chondrocytes alter subchondral bone osteoblast differentiation via MAPK signalling pathway involving ERK1/2. Bone 46, 226–235 (2010).
Amiable, N. et al. Proteinase-activated receptor (PAR)-2 activation impacts bone resorptive properties of human osteoarthritic subchondral bone osteoblasts. Bone 44, 1143–1150 (2009).
Ferrell, W. R., Kelso, E. B., Lockhart, J. C., Plevin, R. & McInnes, I. B. Protease-activated receptor 2: a novel pathogenic pathway in a murine model of osteoarthritis. Ann. Rheum. Dis. 69, 2051–2054 (2010).
Acknowledgements
R. J. Lories is the recipient of a post-doctoral fellowship from the Flanders Research Foundation (FWO Vlaanderen). Research on osteoarthritis in the authors' laboratory is supported by FWO Vlaanderen, a GOA grant from KU Leuven, and Translational Research in Europe—Applied Technologies for Osteoarthritis (TREAT-OA), a large collaborative FP7 project supported by the European Union.
Author information
Authors and Affiliations
Contributions
R. J. Lories and F. P. Luyten contributed equally to all aspects of preparation of this manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Lories, R., Luyten, F. The bone–cartilage unit in osteoarthritis. Nat Rev Rheumatol 7, 43–49 (2011). https://doi.org/10.1038/nrrheum.2010.197
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrrheum.2010.197
This article is cited by
-
Prevalence and correlation with sex, age, and dental status of bone apposition at the mandibular angle and radiographic alterations of the temporomandibular joints: a retrospective observational study in an adult Swiss population
BMC Oral Health (2024)
-
Total hip arthroplasty for adult patients with hip arthritis in Jordan: clinical profiles and patient characteristics
International Orthopaedics (2024)
-
Multifunctional hydrogels: advanced therapeutic tools for osteochondral regeneration
Biomaterials Research (2023)
-
IgSF11 deficiency alleviates osteoarthritis in mice by suppressing early subchondral bone changes
Experimental & Molecular Medicine (2023)
-
Study on the poroelastic behaviors of the defected osteochondral unit
Medical & Biological Engineering & Computing (2023)