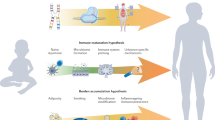
Abstract
Systemic juvenile idiopathic arthritis (sJIA) has long been recognized as unique among childhood arthritides, because of its distinctive clinical and epidemiological features, including an association with macrophage activation syndrome. Here, we summarize research into sJIA pathogenesis. The triggers of disease are unknown, although infections are suspects. Once initiated, sJIA seems to be driven by innate proinflammatory cytokines. Endogenous Toll-like receptor ligands, including S100 proteins, probably synergize with cytokines to perpetuate inflammation. These and other findings support the hypothesis that sJIA is an autoinflammatory condition. Indeed, IL-1 is implicated as a pivotal cytokine, but the source of excess IL-1 activity remains obscure and the role of IL-1 in chronic arthritis is less clear. Another hypothesis is that a form of hemophagocytic lymphohistiocytosis underlies sJIA, with varying degrees of its expression across the spectrum of disease. Alternatively, sJIA with MAS might be a genetically distinct subtype. Yet another hypothesis proposes that inadequate downregulation of immune activation is central to sJIA, supporting evidence for which includes 'alternative activation' of monocyte and macrophages and possible deficiencies in IL-10 and T regulatory cells. Some altered immune phenotypes persist during clinically inactive disease, which suggests that this stage might represent compensated inflammation. Despite much progress being made, many questions remain, providing fertile ground for future research.
Key Points
-
The contribution of innate immunity to systemic juvenile idiopathic arthritis (sJIA) is prominent, supporting the classification of sJIA as an autoinflammatory disorder
-
Available data suggest that sJIA is a multigenic disease, and that sJIA with macrophage activation syndrome (MAS) could represent a genetically distinct disease subtype
-
IL-1β is a critical proinflammatory cytokine in early sJIA, whereas arthritis in chronic persistent sJIA is possibly driven by other mediators
-
During active disease, mediators of both inflammatory and anti-inflammatory pathways are detected; among the latter are monocyte/macrophages with features of 'alternative activation'
-
It is possible that clinically inactive disease (with no medication) represents a state of compensated inflammation rather than the absence of immune activity
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Petty, R. E. et al. International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton, 2001. J. Rheumatol. 31, 390–392 (2004).
Singh-Grewal, D., Schneider, R., Bayer, N. & Feldman, B. M. Predictors of disease course and remission in systemic juvenile idiopathic arthritis: significance of early clinical and laboratory features. Arthritis Rheum. 54, 1595–1601 (2006).
Sandborg, C. et al. Candidate early predictors for progression to joint damage in systemic juvenile idiopathic arthritis. J. Rheumatol. 33, 2322–2329 (2006).
Spiegel, L. R. et al. Early predictors of poor functional outcome in systemic-onset juvenile rheumatoid arthritis: a multicenter cohort study. Arthritis Rheum. 43, 2402–2409 (2000).
Sawhney, S. & Magalhães, C. Paediatric rheumatology—a global perspective. Best Pract. Res. Clin. Rheumatol. 20, 201–221 (2006).
Fujikawa, S. & Okuni, M. Clinical analysis of 570 cases with juvenile rheumatoid arthritis: results of a nationwide retrospective survey in Japan. Acta Paediatr. Jpn 39, 245–249 (1997).
Deane, S., Selmi, C., Teuber, S. & Gershwin, M. E. Macrophage activation syndrome in autoimmune disease. Int. Arch. Allergy Immunol. 153, 109–120 (2010).
Grom, A. Natural killer cell dysfunction: A common pathway in systemic-onset juvenile rheumatoid arthritis, macrophage activation syndrome, and hemophagocytic lymphohistiocytosis? Arthritis Rheum. 50, 689–698 (2004).
Sawhney, S., Woo, P. & Murray, K. J. Macrophage activation syndrome: a potentially fatal complication of rheumatic disorders. Arch. Dis. Child. 85, 421–426 (2001).
Behrens, E. M., Beukelman, T., Paessler, M. & Cron, R. Q. Occult macrophage activation syndrome in patients with systemic juvenile idiopathic arthritis. J. Rheumatol. 34, 1133–1138 (2007).
Bleesing, J. et al. The diagnostic significance of soluble CD163 and soluble interleukin-2 receptor α-chain in macrophage activation syndrome and untreated new-onset systemic juvenile idiopathic arthritis. Arthritis Rheum. 56, 965–971 (2007).
Lindsley, C. B. Seasonal variation in systemic onset juvenile rheumatoid arthritis. Arthritis Rheum. 30, 838–839 (1987).
Oen, K., Fast, M. & Postl, B. Epidemiology of juvenile rheumatoid arthritis in Manitoba, Canada, 1975–1992: cycles in incidence. J. Rheumatol. 22, 745–750 (1995).
Uziel, Y. et al. Seasonal variation in systemic onset juvenile rheumatoid arthritis in Israel. J. Rheumatol. 26, 1187–1189 (1999).
Masters, S., Simon, A., Aksentijevich, I. & Kastner, D. Horror autoinflammaticus: the molecular pathophysiology of autoinflammatory disease. Ann. Rev. Immunol. 27, 621–668 (2009).
Rigante, D. et al. First report of macrophage activation syndrome in hyperimmunoglobulinemia D with periodic fever syndrome. Arthritis Rheum. 56, 658–661 (2007).
Nepom, B. S. & Glass, D. N. Juvenile rheumatoid arthritis and HLA: report of the Park City III workshop. J. Rheumatol. Suppl. 33, 70–74 (1992).
Date, Y. et al. Identification of a genetic risk factor for systemic juvenile rheumatoid arthritis in the 5′-flanking region of the TNFα gene and HLA genes. Arthritis Rheum. 42, 2577–2582 (1999).
Fishman, D. et al. The effect of novel polymorphisms in the interleukin-6 (IL-6) gene on IL-6 transcription and plasma IL-6 levels, and an association with systemic-onset juvenile chronic arthritis. J. Clin. Invest. 102, 1369–1376 (1998).
Ogilvie, E. M. et al. The -174G allele of the interleukin-6 gene confers susceptibility to systemic arthritis in children: a multicenter study using simplex and multiplex juvenile idiopathic arthritis families. Arthritis Rheum. 48, 3202–3206 (2003).
Fife, M. S. et al. Novel IL10 gene family associations with systemic juvenile idiopathic arthritis. Arthritis Res. Ther. 8, R148 (2006).
Moller, J. et al. IL10 promoter polymorphisms are associated with systemic onset juvenile idiopathic arthritis (SoJIA). Clin. Exp. Rheumatol. 28, 912–918 (2010).
Donn, R. P., Shelley, E., Ollier, W. E. & Thomson, W. A novel 5′-flanking region polymorphism of macrophage migration inhibitory factor is associated with systemic-onset juvenile idiopathic arthritis. Arthritis Rheum. 44, 1782–1785 (2001).
De Benedetti, F. et al. Functional and prognostic relevance of the −173 polymorphism of the macrophage migration inhibitory factor gene in systemic-onset juvenile idiopathic arthritis. Arthritis Rheum. 48, 1398–1407 (2003).
Stock, C. J. et al. Comprehensive association study of genetic variants in the IL-1 gene family in systemic juvenile idiopathic arthritis. Genes Immun. 9, 349–357 (2008).
Lamb, R., Thomson, W., Ogilvie, E. & Donn, R. Positive association of SLC26A2 gene polymorphisms with susceptibility to systemic-onset juvenile idiopathic arthritis. Arthritis Rheum. 56, 1286–1291 (2007).
Gattorno, M. et al. The pattern of response to anti-interleukin-1 treatment distinguishes two subsets of patients with systemic-onset juvenile idiopathic arthritis. Arthritis Rheum. 58, 1505–1515 (2008).
Day, T. G. et al. Autoinflammatory genes and susceptibility to psoriatic juvenile idiopathic arthritis. Arthritis Rheum. 58, 2142–2146 (2008).
Ayaz, N. A. et al. MEFV mutations in systemic onset juvenile idiopathic arthritis. Rheumatology (Oxford) 48, 23–25 (2009).
Fall, N. et al. Gene expression profiling of peripheral blood from patients with untreated new-onset systemic juvenile idiopathic arthritis reveals molecular heterogeneity that may predict macrophage activation syndrome. Arthritis Rheum. 56, 3793–3804 (2007).
Macaubas, C. et al. Distribution of circulating cells in systemic juvenile idiopathic arthritis across disease activity states. Clin. Immunol. 134, 206–216 (2010).
Hinze, C. et al. Immature cell populations and an erythropoiesis gene-expression signature in systemic juvenile idiopathic arthritis: implications for pathogenesis. Arthritis Res. Ther. 12, R123 (2010).
Pascual, V., Allantaz, F., Arce, E., Punaro, M. & Banchereau, J. Role of interleukin-1 (IL-1) in the pathogenesis of systemic onset juvenile idiopathic arthritis and clinical response to IL-1 blockade. J. Exp. Med. 201, 1479–1486 (2005).
Ogilvie, E. M., Khan, A., Hubank, M., Kellam, P. & Woo, P. Specific gene expression profiles in systemic juvenile idiopathic arthritis. Arthritis Rheum. 56, 1954–1965 (2007).
Barnes, M. et al. Subtype-specific peripheral blood gene expression profiles in recent-onset juvenile idiopathic arthritis. Arthritis Rheum. 60, 2102–2112 (2009).
Allantaz, F. et al. Blood leukocyte microarrays to diagnose systemic onset juvenile idiopathic arthritis and follow the response to IL-1 blockade. J. Exp. Med. 204, 2131–2144 (2007).
Quartier, P. et al. A multicentre, randomised, double-blind, placebo-controlled trial with the interleukin-1 receptor antagonist anakinra in patients with systemic-onset juvenile idiopathic arthritis (ANAJIS trial). Ann. Rheum. Dis. 70, 747–754 (2011).
Wilson, D. et al. Follistatin-like protein 1 is a mesenchyme-derived inflammatory protein and may represent a biomarker for systemic-onset juvenile rheumatoid arthritis. Arthritis Rheum. 62, 2510–2516 (2010).
Ling, X. et al. Plasma profiles in active systemic juvenile idiopathic arthritis: biomarkers and biological implications. Proteomics 10, 4415–4430 (2010).
Verbsky, J. & White, A. Effective use of the recombinant interleukin 1 receptor antagonist anakinra in therapy resistant systemic onset juvenile rheumatoid arthritis. J. Rheumatol. 31, 2071–2075 (2004).
Irigoyen, P. I., Olson, J., Hom, C. & Ilowite, N. T. Treatment of systemic onset juvenile rheumatoid arthritis with anakinra. Arthritis Rheum. 50, S437 (2004).
Henrickson, M. Efficacy of anakinra in refractory systemic arthritis. Arthritis Rheum. 50, S438 (2004).
Muzaffer, M. A. et al. Differences in the profiles of circulating levels of soluble tumor necrosis factor receptors and interleukin 1 receptor antagonist reflect the heterogeneity of the subgroups of juvenile rheumatoid arthritis. J. Rheumatol. 29, 1071–1078 (2002).
Nigrovic, P. et al. Anakinra as first-line disease modifying therapy in systemic juvenile idiopathic arthritis: report of forty-six patients from an international multicenter series. Arthritis Rheum. 63, 545–555 (2010).
Tassi, S. et al. Altered redox state of monocytes from cryopyrin-associated periodic syndromes causes accelerated IL-1β secretion. Proc. Natl Acad. Sci. USA 107, 9789–9794 (2010).
van den Ham, H.-J., de Jager, W., Bijlsma, J. W. J., Prakken, B. J. & de Boer, R. J. Differential cytokine profiles in juvenile idiopathic arthritis subtypes revealed by cluster analysis. Rheumatology (Oxford) 48, 899–905 (2009).
Guma, M. et al. Caspase 1-independent activation of interleukin-1β in neutrophil-predominant inflammation. Arthritis Rheum. 60, 3642–3650 (2009).
Joosten, L. A. B. et al. Inflammatory arthritis in caspase 1 gene-deficient mice: contribution of proteinase 3 to caspase 1-independent production of bioactive interleukin-1 β. Arthritis Rheum. 60, 3651–3662 (2009).
Metkar, S. S. et al. Human and mouse granzyme A induce a proinflammatory cytokine response. Immunity 29, 720–733 (2008).
Frosch, M. et al. The myeloid-related proteins 8 and 14 complex, a novel ligand of Toll-like receptor 4, and interleukin-1β form a positive feedback mechanism in systemic-onset juvenile idiopathic arthritis. Arthritis Rheum. 60, 883–891 (2009).
Wittkowski, H. et al. S100A12 is a novel molecular marker differentiating systemic-onset juvenile idiopathic arthritis from other causes of fever of unknown origin. Arthritis Rheum. 58, 3924–3931 (2008).
de Benedetti, F. et al. Correlation of serum interleukin-6 levels with joint involvement and thrombocytosis in systemic juvenile rheumatoid arthritis. Arthritis Rheum. 34, 1158–1163 (1991).
de Benedetti, F. et al. Effect of IL-6 on IGF binding protein-3: a study in IL-6 transgenic mice and in patients with systemic juvenile idiopathic arthritis. Endocrinology 142, 4818–4826 (2001).
Cazzola, M. et al. Defective iron supply for erythropoiesis and adequate endogenous erythropoietin production in the anemia associated with systemic-onset juvenile chronic arthritis. Blood 87, 4824–4830 (1996).
Pignatti, P. et al. Abnormal regulation of interleukin 6 in systemic juvenile idiopathic arthritis. J. Rheumatol. 28, 1670–1676 (2001).
Muller, K., Herner, E. B., Stagg, A., Bendtzen, K. & Woo, P. Inflammatory cytokines and cytokine antagonists in whole blood cultures of patients with systemic juvenile chronic arthritis. Rheumatology (Oxford) 37, 562–569 (1998).
Bradshaw, E. M. et al. Monocytes from patients with type 1 diabetes spontaneously secrete proinflammatory cytokines inducing TH17 cells. J. Immunol. 183, 4432–4439 (2009).
Yokota, S. et al. Efficacy and safety of tocilizumab in patients with systemic-onset juvenile idiopathic arthritis: a randomised, double-blind, placebo-controlled, withdrawal phase III trial. Lancet 371, 998–1006 (2008).
De Benedetti, F. et al. Tocilizumab in patients with systemic juvenile idiopathic arthritis: efficacy data from the placebo-controlled 12-week part of the phase 3 TENDER trial. Arthritis Rheum. 62, S596 (2010).
Nakajima, S. et al. Improvement of reduced serum cartilage oligomeric matrix protein levels in systemic juvenile idiopathic arthritis patients treated with the anti-interleukin-6 receptor monoclonal antibody tocilizumab. Mod. Rheumatol. 19, 42–46 (2009).
Sarma, P. K., Misra, R. & Aggarwal, A. Elevated serum receptor activator of NFκB ligand (RANKL), osteoprotegerin (OPG), matrix metalloproteinase (MMP)3, and ProMMP1 in patients with juvenile idiopathic arthritis. Clin. Rheumatol. 27, 289–294 (2008).
Silacci, P. et al. Interleukin (IL)-6 and its soluble receptor induce TIMP-1 expression in synoviocytes and chondrocytes, and block IL-1-induced collagenolytic activity. J. Biol. Chem. 273, 13625–13629 (1998).
Ling, X. et al. Urine peptidomic and targeted plasma protein analyses in the diagnosis and monitoring of systemic juvenile idiopathic arthritis. Clin. Proteomics 6, 175–193 (2010).
Kossakowska, A. E. et al. Interleukin-6 regulation of matrix metalloproteinase (MMP-2 and MMP-9) and tissue inhibitor of metalloproteinase (TIMP-1) expression in malignant non-Hodgkin's lymphomas. Blood 94, 2080–2089 (1999).
de Jager, W. et al. Blood and synovial fluid cytokine signatures in patients with juvenile idiopathic arthritis: a cross-sectional study. Ann. Rheum. Dis. 66, 589–598 (2007).
Takahashi, A. et al. The role of heme oxygenase-1 in systemic-onset juvenile idiopathic arthritis. Mod. Rheumatol. 19, 302–308 (2009).
Martinez, F., Sica, A., Mantovani, A. & Locati, M. Macrophage activation and polarization. Front. Biosci. 13, 453–461 (2008).
Roca, H. et al. CCL2 and interleukin-6 promote survival of human CD11b+ peripheral blood mononuclear cells and induce M2-type macrophage polarization. J. Biol. Chem. 284, 34342–34354 (2009).
Srivastava, S. et al. Monocytes are resistant to apoptosis in systemic juvenile idiopathic arthritis. Clin. Immunol. 136, 257–268 (2010).
Porta, C. et al. Tolerance and M2 (alternative) macrophage polarization are related processes orchestrated by p50 nuclear factor κB. Proc. Natl Acad. Sci. USA 106, 14978–14983 (2009).
Kristiansen, M. et al. Identification of the haemoglobin scavenger receptor. Nature 409, 198–201 (2001).
Avcin, T., Tse, S. M. L., Schneider, R., Ngan, B. & Silverman, E. Macrophage activation syndrome as the presenting manifestation of rheumatic diseases in childhood. J. Pediatrics 148, 683–686 (2006).
Schaer, D. et al. Soluble hemoglobin-haptoglobin scavenger receptor CD163 as a lineage-specific marker in the reactive hemophagocytic syndrome. Eur. J. Haematol. 74, 6–10 (2005).
de Kleer. I. et al. Autologous stem cell transplantation for autoimmunity induces immunologic self-tolerance by reprogramming autoreactive T cells and restoring the CD4+CD25+ immune regulatory network. Blood 107, 1696–1702 (2006).
Nistala, K. et al. Interleukin-17-producing T cells are enriched in the joints of children with arthritis, but have a reciprocal relationship to regulatory T cell numbers. Arthritis Rheum. 58, 875–887 (2008).
Olivito, B. et al. TH17 transcription factor RORC2 is inversely correlated with FOXP3 expression in the joints of children with juvenile idiopathic arthritis. J. Rheumatol. 36, 2017–2024 (2009).
Agarwal, S., Misra, R. & Aggarwal, A. Interleukin 17 levels are increased in juvenile idiopathic arthritis synovial fluid and induce synovial fibroblasts to produce proinflammatory cytokines and matrix metalloproteinases. J. Rheumatol. 35, 515–519 (2008).
Peck, A. & Mellins, E. Breaking old paradigms: TH17 cells in autoimmune arthritis. Clin. Immunol. 132, 295–304 (2009).
Manel, N., Unutmaz, D. & Littman, D. R. The differentiation of human TH17 cells requires transforming growth factor-β and induction of the nuclear receptor RORγt. Nat. Immunol. 9, 641–649 (2008).
Toh, M.-L. et al. Role of interleukin 17 in arthritis chronicity through survival of synoviocytes via regulation of synoviolin expression. PLoS ONE 5, e13416 (2010).
Koenders, M. et al. Interleukin-1 drives pathogenic TH17 cells during spontaneous arthritis in interleukin-1 receptor antagonist-deficient mice. Arthritis Rheum. 58, 3461–3470 (2008).
Donn, R. et al. Genetic loci contributing to hemophagocytic lymphohistiocytosis do not confer susceptibility to systemic-onset juvenile idiopathic arthritis. Arthritis Rheum. 58, 869–874 (2008).
Zhang, K. et al. Macrophage activation syndrome in patients with systemic juvenile idiopathic arthritis is associated with MUNC13-4 polymorphisms. Arthritis Rheum. 58, 2892–2896 (2008).
Vastert, S. et al. Mutations in the perforin gene can be linked to macrophage activation syndrome in patients with systemic onset juvenile idiopathic arthritis. Rheumatology (Oxford) 49, 441–449 (2010).
Yanagimachi M. et al. Association of IRF5 polymorphisms with susceptibility to macrophage activation syndrome in patients with juvenile idiopathic arthritis. J. Rheumatol. 38, 769–774 (2011).
Shimizu, M. et al. Distinct cytokine profiles of systemic-onset juvenile idiopathic arthritis-associated macrophage activation syndrome with particular emphasis on the role of interleukin-18 in its pathogenesis. Rheumatology (Oxford) 49, 1645–1653 (2010).
Imagawa, T. Differences between systemic onset juvenile idiopathic arthritis and macrophage activation syndrome from the standpoint of the proinflammatory cytokine profiles. Arthritis Rheum. 50, S92 (2004).
Billiau, A. D., Roskams, T., Van Damme-Lombaerts, R., Matthys, P. & Wouters, C. Macrophage activation syndrome: characteristic findings on liver biopsy illustrating the key role of activated, IFN-γ-producing lymphocytes and IL-6- and TNF-α-producing macrophages. Blood 105, 1648–1651 (2005).
Bruck, N. et al. Rapid and sustained remission of systemic juvenile idiopathic arthritis-associated macrophage activation syndrome through treatment with anakinra and corticosteroids. J. Clin. Rheum. 17, 23–27 (2011).
Filipovich, A. Hemophagocytic lymphohistiocytosis and other hemophagocytic disorders. Immunol. Allergy Clin. North Am. 28, 293–313, viii (2008).
Voskoboinik, I., Smyth, M. & Trapani, J. Perforin-mediated target-cell death and immune homeostasis. Nat. Rev. Immunol. 6, 940–952 (2006).
Wulffraat, N. M., Rijkers, G. T., Elst, E., Brooimans, R. & Kuis, W. Reduced perforin expression in systemic juvenile idiopathic arthritis is restored by autologous stem-cell transplantation. Rheumatology (Oxford) 42, 375–379 (2003).
Grom, A. A. et al. Natural killer cell dysfunction in patients with systemic-onset juvenile rheumatoid arthritis and macrophage activation syndrome. J. Pediatrics 142, 292–296 (2003).
Villanueva, J. et al. Natural killer cell dysfunction is a distinguishing feature of systemic onset juvenile rheumatoid arthritis and macrophage activation syndrome. Arthritis Res. Ther. 7, R30–R37 (2005).
de Jager, W. et al. Defective phosphorylation of interleukin-18 receptor β causes impaired natural killer cell function in systemic-onset juvenile idiopathic arthritis. Arthritis Rheum. 60, 2782–2793 (2009).
Bufler, P. et al. A complex of the IL-1 homologue IL-1F7b and IL-18-binding protein reduces IL-18 activity. Proc. Natl Acad. Sci. USA 99, 13723–13728 (2002).
Mazodier, K. et al. Severe imbalance of IL-18/IL-18BP in patients with secondary hemophagocytic syndrome. Blood 106, 3483–3489 (2005).
Nold-Petry, C. A. et al. Increased cytokine production in interleukin-18 receptor α-deficient cells is associated with dysregulation of suppressors of cytokine signaling. J. Biol. Chem. 284, 25900–25911 (2009).
Sugimoto, T. et al. Interleukin 18 acts on memory T helper cells type 1 to induce airway inflammation and hyperresponsiveness in a naive host mouse. J. Exp. Med. 199, 535–545 (2004).
Maeno, N. et al. Increased interleukin-18 expression in bone marrow of a patient with systemic juvenile idiopathic arthritis and unrecognized macrophage-activation syndrome. Arthritis Rheum. 50, 1935–1938 (2004).
Coffey, A. J. et al. Host response to EBV infection in X-linked lymphoproliferative disease results from mutations in an SH2-domain encoding gene. Nat. Genet. 20, 129–135 (1998).
Recalcati, S. et al. Differential regulation of iron homeostasis during human macrophage polarized activation. Eur. J. Immunol. 40, 824–835 (2010).
Lukina, E. A., Levina, A. A., Mokeeva, R. A. & YuN, T. The diagnostic significance of serum ferritin indices in patients with malignant and reactive histiocytosis. Br. J. Haematol. 83, 326–329 (1993).
Behrens, E. M. et al. Repeated Toll-like receptor 9 stimulation results in macrophage activation syndrome-like disease in mice. J. Clin. Invest. (in press).
Nguyen, K. D. et al. Serum amyloid A overrides TREG anergy via monocyte-dependent and TREG-intrinsic, SOCS3-associated pathways. Blood 117, 3793–3798 (2011).
Smith, A. M. et al. Disordered macrophage cytokine secretion underlies impaired acute inflammation and bacterial clearance in Crohn's disease. J. Exp. Med. 206, 1883–1897 (2009).
Scott, J. P., Gerber, P., Maryjowski, M. C. & Pachman, L. M. Evidence for intravascular coagulation in systemic onset, but not polyarticular, juvenile rheumatoid arthritis. Arthritis Rheum. 28, 256–261 (1985).
Bloom, B., Toyoda, M., Petrosian, A. & Jordan, S. Anti-endothelial cell antibodies are prevalent in juvenile idiopathic arthritis: implications for clinical disease course and pathogenesis. Rheumatol. Int. 27, 655–660 (2007).
Gabay, C., Lamacchia, C. & Palmer, G. IL-1 pathways in inflammation and human diseases. Nat. Rev. Rheumatol. 6, 232–241 (2010).
Church, L., Cook, G. & McDermott, M. Primer: inflammasomes and interleukin 1β in inflammatory disorders. Nat. Clin. Pract. Rheumatol. 4, 34–42 (2008).
Gattorno, M. et al. Pattern of interleukin-1β secretion in response to lipopolysaccharide and ATP before and after interleukin-1 blockade in patients with CIAS1 mutations. Arthritis Rheum. 56, 3138–3148 (2007).
Srikrishna, G. et al. Two proteins modulating transendothelial migration of leukocytes recognize novel carboxylated glycans on endothelial cells. J. Immunol. 166, 4678–4688 (2001).
Newton, R. A. & Hogg, N. The human S100 protein MRP-14 is a novel activator of the β2 integrin Mac-1 on neutrophils. J. Immunol. 160, 1427–1435 (1998).
Martinez, F. O., Helming, L. & Gordon, S. Alternative activation of macrophages: an immunologic functional perspective. Ann. Rev. Immunol. 27, 451–483 (2009).
El Chartouni, C., Schwarzfischer, L. & Rehli, M. Interleukin-4 induced interferon regulatory factor (Irf) 4 participates in the regulation of alternative macrophage priming. Immunobiology 215, 821–825 (2010).
Krausgruber, T. et al. IRF5 promotes inflammatory macrophage polarization and TH1–TH17 responses. Nat. Immunol. 12, 231–238 (2011).
Acknowledgements
C. P. Vega, University of California, Irvine, CA, is the author of and is solely responsible for the content of the learning objectives, questions and answers of the Medscape, LLC-accredited continuing medical education activity associated with this article.
Author information
Authors and Affiliations
Contributions
All authors contributed equally to researching data for the article, providing substantial contributions to discussions of content and writing the article. E. D. Mellins reviewed and edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
E. D. Mellins has acted as a consultant for Genentech, and A. A. Grom has acted as a consultant for Novartis. C. Macaubas, the journal Chief Editor J. Buckland and the CME questions author C. P. Vega declare no competing interests.
Rights and permissions
About this article
Cite this article
Mellins, E., Macaubas, C. & Grom, A. Pathogenesis of systemic juvenile idiopathic arthritis: some answers, more questions. Nat Rev Rheumatol 7, 416–426 (2011). https://doi.org/10.1038/nrrheum.2011.68
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrrheum.2011.68
This article is cited by
-
Establishment and analysis of a novel diagnostic model for systemic juvenile idiopathic arthritis based on machine learning
Pediatric Rheumatology (2024)
-
Proceedings from the 4th NextGen Therapies for SJIA and MAS virtual symposium held February 13–14, 2022
Pediatric Rheumatology (2024)
-
Diagnosis and Management of the Systemic Juvenile Idiopathic Arthritis Patient with Emerging Lung Disease
Pediatric Drugs (2023)
-
A review of the pleiotropic actions of the IFN-inducible CXC chemokine receptor 3 ligands in the synovial microenvironment
Cellular and Molecular Life Sciences (2023)
-
Glucose metabolism in systemic juvenile idiopathic arthritis
Pediatric Rheumatology (2022)