Abstract
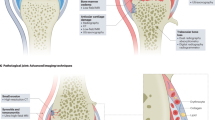
Molecular and multimodal imaging procedures that complement the use of existing anatomical modalities for the diagnosis and monitoring of rheumatoid arthritis (RA) have undergone substantial developmental advances. These techniques have the potential to greatly improve the management of patients with RA through early diagnosis and maximization of the newly available opportunities for early therapeutic intervention. Quantitative, noninvasive monitoring of biomarkers of the molecular events induced during the onset of RA could be used to guide the initial selection of therapy and for assessment of early therapeutic responses. Biomolecular imaging techniques that can reveal the pathophysiological features of RA—including infrared thermography, near-infrared molecular imaging, and PET—are being used to investigate the earliest cellular and biochemical inflammatory events in the development of the disease. Noninvasive imaging of abnormal specific molecular events in early RA could enable early targeted intervention that could be tailored to optimize patient responses before destructive anatomical changes occur. In this Review, we summarize new advances in biomolecular imaging techniques, with an emphasis on their current state of development in terms of the management of RA.
Key Points
-
Biomolecular imaging can detect specific molecular events that are associated with the onset and progression of RA
-
Thermography can be used to detect and quantitatively assess clinically meaningful changes in arthritic joints
-
Near-infrared imaging can detect a broad spectrum of specific enzymatic activities, including local levels of caspase or cathepsin activity
-
PET imaging is a highly specific and sensitive technique that can be used to assess biochemical processes, such as cell metabolism, angiogenesis and apoptosis
-
Noninvasive whole-body analysis of specific molecular events during the onset of RA could facilitate early intervention and the use of tailored therapies to optimize the responses of individual patients
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Brennan, P. et al. A simple algorithm to predict the development of radiological erosions in patients with early rheumatoid arthritis: prospective cohort study. BMJ 313, 471–476 (1996).
Klarlund, M. et al. Magnetic resonance imaging, radiography, and scintigraphy of the finger joints: one year follow up of patients with early arthritis. Ann. Rheum. Dis. 59, 521–528 (2000).
McQueen, F. M. et al. Magnetic resonance imaging of the wrist in early rheumatoid arthritis reveals progression of erosions despite clinical improvement. Ann. Rheum. Dis. 58, 156–163 (1999).
McQueen, F. M. et al. What is the fate of erosions in early rheumatoid arthritis? Tracking individual lesions using x rays and magnetic resonance imaging over the first two years of disease. Ann. Rheum. Dis. 60, 859–868 (2001).
Kokkonen, H. et al. Up-regulation of cytokines and chemokines predates the onset of rheumatoid arthritis. Arthritis Rheum. 62, 383–391 (2010).
Davila, L. & Ranganathan, P. Pharmacogenetics: implications for therapy in rheumatic diseases. Nat. Rev. Rheumatol. 7, 537–550 (2011).
Devereaux, M. D., Parr, G. R., Thomas, D. P. & Hazleman, B. L. Disease activity indexes in rheumatoid arthritis; a prospective, comparative study with thermography. Ann. Rheum. Dis. 44, 434–437 (1985).
Spadling, S. J. et al. Three-dimensional and thermal surface imaging produces reliable measures of joint shape and temperature: a potential tool for quantifying arthritis. Arthritis Res. Ther. 10, R10 (2008).
Fischer, T. et al. Detection of rheumatoid arthritis using non-specific contrast enhanced fluorescence imaging. Acad. Radiol. 17, 375–381 (2010).
Prapavat, V. et al. The development of a finger joint phantom for the optical stimulation of early inflammatory rheumatic changes [German]. Biomed. Tech. (Berl.) 42, 319–326 (1997).
Scheel, A. K. et al. Assessment of proximal finger joint inflammation in patients with rheumatoid arthritis, using a novel laser-based imaging technique. Arthritis Rheum. 46, 1177–1184 (2002).
Hielscher, A. H. et al. Sagittal laser optical tomography for imaging of rheumatoid finger joints. Phys. Med. Biol. 49, 1147–1163 (2004).
Scheel, A. K. et al. First clinical evaluation of sagittal laser optical tomography for detection of synovitis in arthritic finger joints. Ann. Rheum. Dis. 64, 239–245 (2005).
Klose, C. D., Klose, A. D., Netz, U., Beuthan, J. & Hielscher, A. H. Multiparameter classifications of optical tomographic images. J. Biomed. Opt. 13, 050503 (2008).
Aswathy, R. G., Yoshida, Y., Maekawa, T. & Kumar, D. S. Near-infrared quantum dots for deep tissue imaging. Anal. Bioanal. Chem. 397, 1417–1435 (2010).
Canvin, J. M. et al. Infrared spectroscopy: shedding light on synovitis in patients with rheumatoid arthritis. Rheumatology (Oxford) 42, 76–82 (2003).
Fischer, T. et al. Assessment of unspecific near-infrared dyes in laser-induced fluorescence imaging of experimental arthritis. Acad. Radiol. 13, 4–13 (2006).
Hansch, A. et al. In vivo imaging of experimental arthritis with near-infrared fluorescence. Arthritis Rheum. 50, 961–967 (2004).
Hansch, A. et al. Diagnosis of arthritis using near-infrared fluorochrome Cy5.5. Invest. Radiol. 39, 626–632 (2004).
Chen, W. T., Mahmood, U., Weissleder, R. & Tung, C. H. Arthritis imaging using a near-infrared fluorescence folate-targeted probe. Arthritis Res. Ther. 7, R310–R317 (2005).
Simon, G. H. et al. Optical imaging of experimental arthritis using allogeneic leukocytes labeled with a near-infrared fluorescent probe. Eur. J. Nucl. Med. Mol. Imaging 33, 998–1006 (2006).
Pogue, B. W. Near-infrared characterization of disease via vascular permeability probes. Acad. Radiol. 13, 1–3 (2006).
Rengel, Y., Ospelt, C. & Gay, S. Proteinases in the joint: clinical relevance of proteinases in joint destruction. Arthritis Res. Ther. 9, 221 (2007).
Ntziachristos, V., Tung, C. H., Bremer, C. & Weissleder, R. Fluorescence molecular tomography resolves protease activity in vivo. Nat. Med. 8, 757–760 (2002).
Wunder, A., Tung, C. H., Müller-Ladner, U., Weissleder, R. & Mahmood, U. In vivo imaging of protease activity in arthritis: a novel approach for monitoring treatment response. Arthritis Rheum. 50, 2459–2465 (2004).
Li, J. et al. Treatment of arthritis by macrophage depletion and immunomodulation: testing an apoptosis-mediated therapy in a humanized death receptor mouse model. Arthritis Rheum. 64, 1098–1109 (2012).
Post, A. M. et al. Imaging cell death with radiolabeled annexin V in an experimental model of rheumatoid arthritis. J. Nucl. Med. 43, 1359–1365 (2002).
Blankenberg, F. G. In vivo detection of apoptosis. J. Nucl. Med. 49 (Suppl. 2), 81S–95S (2008).
Edgington, L. E. et al. Noninvasive optical imaging of apoptosis by caspase-targeted activity-based probes. Nat. Med. 15, 967–973 (2009).
McBride, H. J. Nuclear imaging of autoimmunity: focus on IBD and RA. Autoimmunity 43, 539–549 (2010).
Gotthardt, M., Bleeker-Rovers, C. P., Boerman, O. C. & Oyen, W. J. Imaging of inflammation by PET, conventional scintigraphy, and other imaging techniques. J. Nucl. Med. 51, 1937–1949 (2010).
Sokoloff, L. et al. The [14C]deoxyglucose method for the measurement of local cerebral glucose utilization: theory, procedure, and normal values in the conscious and anesthetized albino rat. J. Neurochem. 28, 897–916 (1977).
Hawkins, R. A. et al. PET cancer evaluations with FDG. J. Nucl. Med. 32, 1555–1558 (1991).
Ju, J. H. et al. Visualization and localization of rheumatoid knee synovitis with FDG-PET/CT images. Clin. Rheumatol. 27 (Suppl. 2), S39–S41 (2008).
Matsui, T. et al. Inflammatory cytokines and hypoxia contribute to 18F-FDG uptake by cells involved in pannus formation in rheumatoid arthritis. J. Nucl. Med. 50, 920–926 (2009).
Basu, S. et al. Novel quantitative techniques for assessing regional and global function and structure based on modern imaging modalities: implications for normal variation, aging and diseased states. Semin. Nucl. Med. 37, 223–239 (2007).
Torigian, D. A. et al. Feasibility and performance of novel software to quantify metabolically active volumes and 3D partial volume corrected SUV and metabolic volumetric products of spinal bone marrow metastases on 18F-FDG-PET/CT. Hell. J. Nucl. Med. 14, 8–14 (2011).
Basu, S. & Alavi, A. Unparalleled contribution of 18F-FDG PET to medicine over 3 decades. J. Nucl. Med. 49, 17N–21N, 37N (2008).
Basu, S. et al. Functional imaging of inflammatory diseases using nuclear medicine techniques. Semin. Nucl. Med. 39, 124–145 (2009).
Vogel, W. V., van Riel, P. L. & Oyen, W. J. FDG-PET/CT can visualise the extent of inflammation in rheumatoid arthritis of the tarsus. Eur. J. Nucl. Med. Mol. Imaging 34, 439 (2007).
Brenner, W. 18F-FDG PET in rheumatoid arthritis: there still is a long way to go. J. Nucl. Med. 45, 927–929 (2004).
Fonseca, A. et al. 18F-FDG PET imaging of rheumatoid articular and extraarticular synovitis. J. Clin. Rheumatol. 14, 307 (2008).
Kubota, K. et al. Whole-body FDG-PET/CT on rheumatoid arthritis of large joints. Ann. Nucl. Med. 23, 783–791 (2009).
Lin, P. W., Liu, R. S., Liou, T. H., Pan, L. C. & Chen, C. H. Correlation between joint [F-18] FDG PET uptake and synovial TNF-α concentration: a study with two rabbit models of acute inflammatory arthritis. Appl. Radiat. Isot. 65, 1221–1226 (2007).
Mountz, J. D. et al. Molecular imaging: new applications for biochemistry. J. Cell. Biochem. Suppl. 39, 162–171 (2002).
Roivainen, A. et al. Use of positron emission tomography with methyl-11C-choline and 2-18F-fluoro-2-deoxy-D-glucose in comparison with magnetic resonance imaging for the assessment of inflammatory proliferation of synovium. Arthritis Rheum. 48, 3077–3084 (2003).
Polisson, R. P. et al. Use of magnetic resonance imaging and positron emission tomography in the assessment of synovial volume and glucose metabolism in patients with rheumatoid arthritis. Arthritis Rheum. 38, 819–825 (1995).
Carey, K. et al. Evolving role of FDG PET imaging in assessing joint disorders: a systematic review. Eur. J. Nucl. Med. Mol. Imaging 38, 1939–1955 (2011).
Beckers, C. et al. Assessment of disease activity in rheumatoid arthritis with 18F-FDG PET. J. Nucl. Med. 45, 956–964 (2004).
Beckers, C. et al. 18F-FDG PET imaging of rheumatoid knee synovitis correlates with dynamic magnetic resonance and sonographic assessments as well as with the serum level of metalloproteinase-3. Eur. J Nucl. Med. Mol. Imaging 33, 275–280 (2006).
Palmer, W. E. et al. Quantification of inflammation in the wrist with gadolinium-enhanced MR imaging and PET with 2-[F-18]-fluoro-2-deoxy-D-glucose. Radiology 196, 647–655 (1995).
Goerres, G. W. et al. F-18 FDG whole-body PET for the assessment of disease activity in patients with rheumatoid arthritis. Clin. Nucl. Med. 31, 386–390 (2006).
Szekanecz, Z., Besenyei, T., Paragh, G. & Koch, A. E. New insights in synovial angiogenesis. Joint Bone Spine 77, 13–19 (2010).
Friedlander, M. et al. Involvement of integrins αvβ3 and αvβ5 in ocular neovascular diseases. Proc. Natl Acad. Sci. USA 93, 9764–9769 (1996).
Sipkins, D. A. et al. Detection of tumor angiogenesis in vivo by αvβ3-targeted magnetic resonance imaging. Nat. Med. 4, 623–626 (1998).
Nakamura, I., Duong le, T., Rodan, S. B. & Rodan, G. A. Involvement of αvβ3 integrins in osteoclast function. J. Bone Miner. Metab. 25, 337–344 (2007).
Wilder, R. L. Integrin αvβ3 as a target for treatment of rheumatoid arthritis and related rheumatic diseases. Ann. Rheum. Dis. 61 (Suppl. 2), ii96–ii99 (2002).
Beer, A. J. et al. PET-based human dosimetry of 18F-galacto-RGD, a new radiotracer for imaging αvβ3 expression. J. Nucl. Med. 47, 763–769 (2006).
Niu, G. & Chen, X. PET Imaging of Angiogenesis. PET Clin. 4, 17–38 (2009).
Leung, K. 4-[18F]Fluorobenzoyl-Phe-Ala-Leu-Gly-Glu-Ala-NH2. Molecular Imaging and Contrast Agent Database [online], (2011).
Zheleznyak, A. et al. Integrin αvβ3 as a PET imaging biomarker for osteoclast number in mouse models of negative and positive osteoclast regulation. Mol. Imaging Biol. http://dx.doi.org/10.1007/s11307-011-0512-4.
Martens, C. L. et al. Peptides which bind to E-selectin and block neutrophil adhesion. J. Biol. Chem. 270, 21129–21136 (1995).
Gaál, J. et al. 99mTc-HMPAO labelled leukocyte scintigraphy in patients with rheumatoid arthritis: a comparison with disease activity. Nucl. Med. Commun. 23, 39–46 (2002).
Barrera, P. et al. Radiolabelled interleukin-1 receptor antagonist for detection of synovitis in patients with rheumatoid arthritis. Rheumatology (Oxford) 39, 870–874 (2000).
Roimicher, L. et al. 99mTc-anti-TNF-α scintigraphy in RA: a comparison pilot study with MRI and clinical examination. Rheumatology (Oxford) 50, 2044–2050 (2011).
Gent, Y. Y. et al. Macrophage positron emission tomography imaging as a biomarker for preclinical rheumatoid arthritis: findings of a prospective pilot study. Arthritis Rheum. 64, 62–66 (2012).
van der Laken, C. J. et al. Noninvasive imaging of macrophages in rheumatoid synovitis using 11C-(R)-PK11195 and positron emission tomography. Arthritis Rheum. 58, 3350–3355 (2008).
Schrigten, D. et al. A new generation of radiofluorinated pyrimidine-2, 4, 6-triones as MMP-targeted radiotracers for positron emission tomography. J. Med. Chem. 55, 223–232 (2012).
Beyer, T. et al. A combined PET/CT scanner for clinical oncology. J. Nucl. Med. 41, 1369–1379 (2000).
Tran, L. et al. CD20 antigen imaging with 124I–rituximab PET/CT in patients with rheumatoid arthritis. Hum. Antibodies 20, 29–35 (2011).
Delso, G. et al. Performance measurements of the Siemens mMR integrated whole-body PET/MR scanner. J. Nucl. Med. 52, 1914–1922 (2011).
Borrero, C. G., Mountz, J. M. & Mountz, J. D. Emerging MRI methods in rheumatoid arthritis. Nat. Rev. Rheumatol. 7, 85–95 (2011).
Kropholler, M. A. et al. Quantification of (R)-[11C]PK11195 binding in rheumatoid arthritis. Eur. J. Nucl. Med. Mol. Imaging 36, 624–631 (2009).
Acknowledgements
J. D. Mountz's research work is funded by grants from the American College of Rheumatology Research and Education Foundation—Within Our Reach: Finding a Cure for Rheumatoid Arthritis campaign, the Alliance for Lupus Research—Target Identification in Lupus program, Veterans Administration Merit Review Grants (1I01BX000600-01), and NIH grants (1AI 071110 and ARRA 3RO1AI71110). The authors thank H. Hsu and J. Li for critical reading of this manuscript, F. Hunter for editorial suggestions and C. Humber and D. M. Frasher for assistance with manuscript preparation.
Author information
Authors and Affiliations
Contributions
All authors researched the data for the article, provided substantial contributions to discussions of the content, wrote the article and undertook review and editing of the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Mountz, J., Alavi, A. & Mountz, J. Emerging optical and nuclear medicine imaging methods in rheumatoid arthritis. Nat Rev Rheumatol 8, 719–728 (2012). https://doi.org/10.1038/nrrheum.2012.148
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrrheum.2012.148
This article is cited by
-
Activatable fluorescent probes for imaging and diagnosis of rheumatoid arthritis
Military Medical Research (2023)
-
Evaluation of the therapeutic potential of the selective p38 MAPK inhibitor Skepinone-L and the dual p38/JNK 3 inhibitor LN 950 in experimental K/BxN serum transfer arthritis
Inflammopharmacology (2019)
-
The Influence of Polysorbate 80 on the Radiochemical Synthesis of a PET Tracer in the FASTlab
Pharmaceutical Research (2015)
-
Mannose receptor (CD206)-mediated imaging in sentinel lymph node localization
Clinical and Translational Imaging (2015)