Abstract
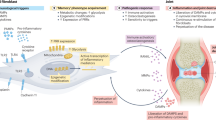
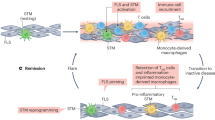
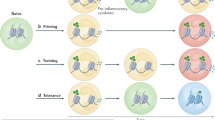
Why do we still have no cure for chronic inflammatory diseases? One reason could be that current therapies are based on the assumption that chronic inflammation is driven by persistent ‘acute’ immune reactions. Here we discuss a paradigm shift by suggesting that beyond these reactions, chronic inflammation is driven by imprinted, pathogenic ‘memory’ cells of the immune system. This rationale is based on the observation that in patients with chronic inflammatory rheumatic diseases refractory to conventional immunosuppressive therapies, therapy-free remission can be achieved by resetting the immune system; that is, by ablating immune cells and regenerating the immune system from stem cells. The success of this approach identifies antigen-experienced and imprinted immune cells as essential and sufficient drivers of inflammation. The ‘dark side’ of immunological memory primarily involves memory plasma cells secreting pathogenic antibodies and memory T lymphocytes secreting pathogenic cytokines and chemokines, but can also involve cells of innate immunity. New therapeutic strategies should address the persistence of these memory cells. Selective targeting of pathogenic immune memory cells could be based on their specificity, which is challenging, or on their lifestyle, which differs from that of protective immune memory cells, in particular for pathogenic T lymphocytes. The adaptations of such pathogenic memory cells to chronic inflammation offers entirely new therapeutic options for their selective ablation and the regeneration of immunological tolerance.
Key points
-
Chronic inflammatory rheumatic diseases are driven by long-lived, adapted memory cells: memory plasma cells, memory B and T lymphocytes and imprinted innate cells.
-
Memory cells have passed all physiological tolerance checkpoints; they are refractory to conventional therapeutic efforts that aim to restore tolerance.
-
Elimination of pathogenic memory cells is a prerequisite for the restoration of immunological tolerance and achievement of therapy-free remission.
-
Analysis of transcriptomes and physiology of pathogenic memory cells has revealed novel therapeutic candidate targets for their selective elimination and suggests that combination therapies could be relevant.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
von Behring, E. Ueber das zustandekommen der Diphtherie-Immunität und der Tetanus-Immunität bei Thieren. (Philipps-Universität Marburg, 1890).
Manz, R. A., Thiel, A. & Radbruch, A. Lifetime of plasma cells in the bone marrow. Nature 388, 133–134 (1997).
Radbruch, A. et al. Competence and competition: the challenge of becoming a long-lived plasma cell. Nat. Rev. Immunol. 6, 741–750 (2006).
Amanna, I. J., Carlson, N. E. & Slifka, M. K. Duration of humoral immunity to common viral and vaccine antigens. N. Engl. J. Med. 357, 1903–1915 (2007).
Slifka, M. K., Antia, R., Whitmire, J. K. & Ahmed, R. Humoral immunity due to long-lived plasma cells. Immunity 8, 363–372 (1998).
Nutt, S. L., Hodgkin, P. D., Tarlinton, D. M. & Corcoran, L. M. The generation of antibody-secreting plasma cells. Nat. Rev. Immunol. 15, 160–171 (2015).
Landsverk, O. J. et al. Antibody-secreting plasma cells persist for decades in human intestine. J. Exp. Med. 214, 309–317 (2017).
Hammarlund, E. et al. Plasma cell survival in the absence of B cell memory. Nat. Commun. 8, 1781 (2017).
Okumura, K., Julius, M. H., Tsu, T. & Herzenberg, L. A. Demonstration that IgG memory is carried by IgG-bearing cells. Eur. J. Immunol. 6, 467–472 (1976).
Rogers, P. R., Dubey, C. & Swain, S. L. Qualitative changes accompany memory T cell generation: faster, more effective responses at lower doses of antigen. J. Immunol. 164, 2338–2346 (2000).
Zinkernagel, R. M. On differences between immunity and immunological memory. Curr. Opin. Immunol. 14, 523–536 (2002).
Tykocinski, L. O. et al. A critical control element for interleukin-4 memory expression in T helper lymphocytes. J. Biol. Chem. 280, 28177–28185 (2005).
Dong, J. et al. Loss of methylation at the IFNG promoter and CNS-1 is associated with the development of functional IFN-γ memory in human CD4+ T lymphocytes. Eur. J. Immunol. 43, 793–804 (2013).
Chang, H. D., Tokoyoda, K. & Radbruch, A. Immunological memories of the bone marrow. Immunol. Rev. 283, 86–98 (2018).
Di Rosa, F. Maintenance of memory T cells in the bone marrow: survival or homeostatic proliferation? Nat. Rev. Immunol. 16, 271 (2016).
Di Rosa, F. & Gebhardt, T. Bone marrow T cells and the integrated functions of recirculating and tissue-resident memory T cells. Front. Immunol. 7, 51 (2016).
Giesecke, C. et al. Tissue distribution and dependence of responsiveness of human antigen-specific memory B cells. J. Immunol. 192, 3091–3100 (2014).
Riedel, R. et al. Discrete populations of isotype-switched memory B lymphocytes are maintained in murine spleen and bone marrow. Nat. Commun. 11, 2570 (2020).
Croft, M., Bradley, L. M. & Swain, S. L. Naive versus memory CD4 T cell response to antigen. Memory cells are less dependent on accessory cell costimulation and can respond to many antigen-presenting cell types including resting B cells. J. Immunol. 152, 2675–2685 (1994).
London, C. A., Lodge, M. P. & Abbas, A. K. Functional responses and costimulator dependence of memory CD4+ T cells. J. Immunol. 164, 265–272 (2000).
Inoue, T., Moran, I., Shinnakasu, R., Phan, T. G. & Kurosaki, T. Generation of memory B cells and their reactivation. Immunol. Rev. 283, 138–149 (2018).
Netea, M. G., Quintin, J. & van der Meer, J. W. Trained immunity: a memory for innate host defense. Cell Host Microbe 9, 355–361 (2011).
Blank, C. U. et al. Defining ‘T cell exhaustion’. Nat. Rev. Immunol. 19, 665–674 (2019).
Wherry, E. J. & Kurachi, M. Molecular and cellular insights into T cell exhaustion. Nat. Rev. Immunol. 15, 486–499 (2015).
Guo, Q. et al. Rheumatoid arthritis: pathological mechanisms and modern pharmacologic therapies. Bone Res. 6, 15 (2018).
Romao, V. C. & Fonseca, J. E. Major challenges in rheumatology: will we ever treat smarter, instead of just harder? Front. Med. 6, 144 (2019).
Mangoni, A. A. et al. Relapse rates after elective discontinuation of anti-TNF therapy in rheumatoid arthritis: a meta-analysis and review of literature. BMC Rheumatol. 3, 10 (2019).
Alexander, T. et al. Depletion of autoreactive immunologic memory followed by autologous hematopoietic stem cell transplantation in patients with refractory SLE induces long-term remission through de novo generation of a juvenile and tolerant immune system. Blood 113, 214–223 (2009).
Alexander, T. et al. Hematopoietic stem cell therapy for autoimmune diseases — clinical experience and mechanisms. J. Autoimmun. 92, 35–46 (2018).
King, C., Ilic, A., Koelsch, K. & Sarvetnick, N. Homeostatic expansion of T cells during immune insufficiency generates autoimmunity. Cell 117, 265–277 (2004).
Burt, R. K. et al. Association of nonmyeloablative hematopoietic stem cell transplantation with neurological disability in patients with relapsing-remitting multiple sclerosis. JAMA 313, 275–284 (2015).
Fagraeus, A. Plasma cellular reaction and its relation to the formation of antibodies in vitro. Nature 159, 499 (1947).
Cooper, M. D., Peterson, R. D. & Good, R. A. Delineation of the thymic and bursal lymphoid systems in the chicken. Nature 205, 143–146 (1965).
Cavelti, P. A. Autoantibodies in rheumatic fever. Proc. Soc. Exp. Biol. Med. 60, 379–381 (1945).
Gear, J. Autoantigens and autoantibodies in the pathogenesis of disease with special refenence to blackwater fever. Trans. R. Soc. Trop. Med. Hyg. 39, 301–314 (1945).
Mackay, I. R. Travels and travails of autoimmunity: a historical journey from discovery to rediscovery. Autoimmun. Rev. 9, A251–258 (2010).
Suurmond, J. & Diamond, B. Autoantibodies in systemic autoimmune diseases: specificity and pathogenicity. J. Clin. Invest. 125, 2194–2202 (2015).
Kouskoff, V. et al. Organ-specific disease provoked by systemic autoimmunity. Cell 87, 811–822 (1996).
Nimmerjahn, F. & Ravetch, J. V. Fcγ receptors as regulators of immune responses. Nat. Rev. Immunol. 8, 34–47 (2008).
Hansen, I. S., Baeten, D. L. P. & den Dunnen, J. The inflammatory function of human IgA. Cell Mol. Life Sci. 76, 1041–1055 (2019).
Kubagawa, H. et al. Functional roles of the IgM Fc receptor in the immune system. Front. Immunol. 10, 945 (2019).
Ochsenbein, A. F. et al. Protective long-term antibody memory by antigen-driven and T help-dependent differentiation of long-lived memory B cells to short-lived plasma cells independent of secondary lymphoid organs. Proc. Natl Acad. Sci. USA 97, 13263–13268 (2000).
Nossal, G. J. Antibody production by single cells. III. The histology of antibody production. Br. J. Exp. Pathol. 40, 301–311 (1959).
Benner, R., Meima, F., van der Meulen, G. M. & van Muiswinkel, W. B. Antibody formation in mouse bone marrow. I. Evidence for the development of plaque-forming cells in situ. Immunology 26, 247–255 (1974).
Manz, R. A., Lohning, M., Cassese, G., Thiel, A. & Radbruch, A. Survival of long-lived plasma cells is independent of antigen. Int. Immunol. 10, 1703–1711 (1998).
Farber, D. L., Netea, M. G., Radbruch, A., Rajewsky, K. & Zinkernagel, R. M. Immunological memory: lessons from the past and a look to the future. Nat. Rev. Immunol. 16, 124–128 (2016).
Hoyer, B. F. et al. Short-lived plasmablasts and long-lived plasma cells contribute to chronic humoral autoimmunity in NZB/W mice. J. Exp. Med. 199, 1577–1584 (2004).
Cheng, Q. et al. Autoantibodies from long-lived ‘memory’ plasma cells of NZB/W mice drive immune complex nephritis. Ann. Rheum. Dis. 72, 2011–2017 (2013).
Cambridge, G. et al. Serologic changes following B lymphocyte depletion therapy for rheumatoid arthritis. Arthritis Rheum. 48, 2146–2154 (2003).
Ferraro, A. J., Drayson, M. T., Savage, C. O. & MacLennan, I. C. Levels of autoantibodies, unlike antibodies to all extrinsic antigen groups, fall following B cell depletion with rituximab. Eur. J. Immunol. 38, 292–298 (2008).
Hiepe, F. et al. Long-lived autoreactive plasma cells drive persistent autoimmune inflammation. Nat. Rev. Rheumatol. 7, 170–178 (2011).
Jonsdottir, T. et al. Treatment of refractory SLE with rituximab plus cyclophosphamide: clinical effects, serological changes, and predictors of response. Ann. Rheum. Dis. 67, 330–334 (2008).
Mumtaz, I. M. et al. Bone marrow of NZB/W mice is the major site for plasma cells resistant to dexamethasone and cyclophosphamide: implications for the treatment of autoimmunity. J. Autoimmun. 39, 180–188 (2012).
Hiepe, F. & Radbruch, A. Plasma cells as an innovative target in autoimmune disease with renal manifestations. Nat. Rev. Nephrol. 12, 232–240 (2016).
Chang, H. D. et al. Pathogenic memory plasma cells in autoimmunity. Curr. Opin. Immunol. 61, 86–91 (2019).
Manz, R. A. & Radbruch, A. Plasma cells for a lifetime? Eur. J. Immunol. 32, 923–927 (2002).
Cassese, G. et al. Plasma cell survival is mediated by synergistic effects of cytokines and adhesion-dependent signals. J. Immunol. 171, 1684–1690 (2003).
Cassese, G. et al. Inflamed kidneys of NZB / W mice are a major site for the homeostasis of plasma cells. Eur. J. Immunol. 31, 2726–2732 (2001).
Starke, C. et al. High frequency of autoantibody-secreting cells and long-lived plasma cells within inflamed kidneys of NZB/W F1 lupus mice. Eur. J. Immunol. 41, 2107–2112 (2011).
Hauser, A. E. et al. Chemotactic responsiveness toward ligands for CXCR3 and CXCR4 is regulated on plasma blasts during the time course of a memory immune response. J. Immunol. 169, 1277–1282 (2002).
Muehlinghaus, G. et al. Regulation of CXCR3 and CXCR4 expression during terminal differentiation of memory B cells into plasma cells. Blood 105, 3965–3971 (2005).
Odendahl, M. et al. Generation of migratory antigen-specific plasma blasts and mobilization of resident plasma cells in a secondary immune response. Blood 105, 1614–1621 (2005).
Mei, H. E. et al. Blood-borne human plasma cells in steady state are derived from mucosal immune responses. Blood 113, 2461–2469 (2009).
Addo, R. K. et al. Single-cell transcriptomes of murine bone marrow stromal cells reveal niche-associated heterogeneity. Eur. J. Immunol. 49, 1372–1379 (2019).
Allen, C. D. et al. Germinal center dark and light zone organization is mediated by CXCR4 and CXCR5. Nat. Immunol. 5, 943–952 (2004).
Cheng, Q. et al. CXCR4-CXCL12 interaction is important for plasma cell homing and survival in NZB/W mice. Eur. J. Immunol. 48, 1020–1029 (2018).
O’Connor, B. P. et al. BCMA is essential for the survival of long-lived bone marrow plasma cells. J. Exp. Med. 199, 91–98 (2004).
Peperzak, V. et al. Mcl-1 is essential for the survival of plasma cells. Nat. Immunol. 14, 290–297 (2013).
Zehentmeier, S. et al. Static and dynamic components synergize to form a stable survival niche for bone marrow plasma cells. Eur. J. Immunol. 44, 2306–2317 (2014).
van Spriel, A. B. et al. The tetraspanin CD37 orchestrates the α4β1 integrin-Akt signaling axis and supports long-lived plasma cell survival. Sci. Signal. 5, ra82 (2012).
Auner, H. W., Beham-Schmid, C., Dillon, N. & Sabbattini, P. The life span of short-lived plasma cells is partly determined by a block on activation of apoptotic caspases acting in combination with endoplasmic reticulum stress. Blood 116, 3445–3455 (2010).
Cornelis, R. et al. Stromal cell-contact dependent PI3K and APRIL induced NF-κB signaling prevent mitochondrial- and ER stress induced death of memory plasma cells. Cell Rep. 32, 107982 (2020).
DiLillo, D. J. et al. Maintenance of long-lived plasma cells and serological memory despite mature and memory B cell depletion during CD20 immunotherapy in mice. J. Immunol. 180, 361–371 (2008).
Manne, C. et al. Salmonella SiiE prevents an efficient humoral immune memory by interfering with IgG+ plasma cell persistence in the bone marrow. Proc. Natl Acad. Sci. USA 116, 7425–7430 (2019).
Xiang, Z. et al. FcγRIIb controls bone marrow plasma cell persistence and apoptosis. Nat. Immunol. 8, 419–429 (2007).
Manz, R., Assenmacher, M., Pfluger, E., Miltenyi, S. & Radbruch, A. Analysis and sorting of live cells according to secreted molecules, relocated to a cell-surface affinity matrix. Proc. Natl Acad. Sci. USA 92, 1921–1925 (1995).
Taddeo, A. et al. Selection and depletion of plasma cells based on the specificity of the secreted antibody. Eur. J. Immunol. 45, 317–319 (2015).
Cheng, Q. et al. Selective depletion of plasma cells in vivo based on the specificity of their secreted antibodies. Eur. J. Immunol. (2019).
Hofmann, K., Clauder, A. K. & Manz, R. A. Targeting B cells and plasma cells in autoimmune diseases. Front. Immunol. 9, 835 (2018).
Alexander, T. et al. The proteasome inhibitior bortezomib depletes plasma cells and ameliorates clinical manifestations of refractory systemic lupus erythematosus. Ann. Rheum. Dis. 74, 1474–1478 (2015).
Alexander, T. et al. Proteasome inhibition with bortezomib induces a therapeutically relevant depletion of plasma cells in SLE but does not target their precursors. Eur. J. Immunol. 48, 1573–1579 (2018).
Ostendorf, L. et al. Targeting CD38 with daratumumab in refractory systemic lupus erythematosus. N. Engl. J. Med. 383, 1149–1155 (2020).
Khodadadi, L. et al. Bortezomib plus continuous B cell depletion results in sustained plasma cell depletion and amelioration of lupus nephritis in NZB/W F1 mice. PLoS One 10, e0135081 (2015).
Taddeo, A. et al. Long-lived plasma cells are early and constantly generated in New Zealand Black/New Zealand White F1 mice and their therapeutic depletion requires a combined targeting of autoreactive plasma cells and their precursors. Arthritis Res. Ther. 17, 39 (2015).
Ellebrecht, C. T. et al. Reengineering chimeric antigen receptor T cells for targeted therapy of autoimmune disease. Science 353, 179–184 (2016).
Macauley, M. S. et al. Antigenic liposomes displaying CD22 ligands induce antigen-specific B cell apoptosis. J. Clin. Invest. 123, 3074–3083 (2013).
Li, R. et al. Proinflammatory GM-CSF-producing B cells in multiple sclerosis and B cell depletion therapy. Sci. Transl. Med. 7, 310ra166 (2015).
Fillatreau, S., Sweenie, C. H., McGeachy, M. J., Gray, D. & Anderton, S. M. B cells regulate autoimmunity by provision of IL-10. Nat. Immunol. 3, 944–950 (2002).
Shen, P. et al. IL-35-producing B cells are critical regulators of immunity during autoimmune and infectious diseases. Nature 507, 366–370 (2014).
Lino, A. C. et al. LAG-3 inhibitory receptor expression identifies immunosuppressive natural regulatory plasma cells. Immunity 49, 120–133.e9 (2018).
Duddy, M. et al. Distinct effector cytokine profiles of memory and naive human B cell subsets and implication in multiple sclerosis. J. Immunol. 178, 6092–6099 (2007).
Ahmed, R. & Gray, D. Immunological memory and protective immunity: understanding their relation. Science 272, 54–60 (1996).
Alp, O. S. & Radbruch, A. The lifestyle of memory CD8+ T cells. Nat. Rev. Immunol. 16, 271 (2016).
McGregor, D. D. & Gowans, J. L. The antibody response of rats depleted of lymphocytes by chronic drainage from the thoracic duct. J. Exp. Med. 117, 303–320 (1963).
Sallusto, F., Lenig, D., Forster, R., Lipp, M. & Lanzavecchia, A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 401, 708–712 (1999).
Mueller, S. N., Gebhardt, T., Carbone, F. R. & Heath, W. R. Memory T cell subsets, migration patterns, and tissue residence. Annu. Rev. Immunol. 31, 137–161 (2013).
Jiang, X. et al. Skin infection generates non-migratory memory CD8+ TRM cells providing global skin immunity. Nature 483, 227–231 (2012).
Mackay, L. K. et al. Long-lived epithelial immunity by tissue-resident memory T (TRM) cells in the absence of persisting local antigen presentation. Proc. Natl Acad. Sci. USA 109, 7037–7042 (2012).
Tokoyoda, K. et al. Professional memory CD4+ T lymphocytes preferentially reside and rest in the bone marrow. Immunity 30, 721–730 (2009).
Okhrimenko, A. et al. Human memory T cells from the bone marrow are resting and maintain long-lasting systemic memory. Proc. Natl Acad. Sci. USA 111, 9229–9234 (2014).
Surh, C. D. & Sprent, J. Homeostasis of naive and memory T cells. Immunity 29, 848–862 (2008).
Di Rosa, F. & Pabst, R. The bone marrow: a nest for migratory memory T cells. Trends Immunol. 26, 360–366 (2005).
Siracusa, F. et al. Maintenance of CD8+ memory T lymphocytes in the spleen but not in the bone marrow is dependent on proliferation. Eur. J. Immunol. 47, 1900–1905 (2017).
Siracusa, F. et al. CD69+ memory T lymphocytes of the bone marrow and spleen express the signature transcripts of tissue-resident memory T lymphocytes. Eur. J. Immunol. 49, 966–968 (2019).
Kumar, B. V. et al. Human tissue-resident memory T cells are defined by core transcriptional and functional signatures in lymphoid and mucosal sites. Cell Rep. 20, 2921–2934 (2017).
Walsh, D. A. et al. The functional requirement for CD69 in establishment of resident memory CD8+ T cells varies with tissue location. J. Immunol. 203, 946–955 (2019).
Shiow, L. R. et al. CD69 acts downstream of interferon-α/β to inhibit S1P1 and lymphocyte egress from lymphoid organs. Nature 440, 540–544 (2006).
Bankovich, A. J., Shiow, L. R. & Cyster, J. G. CD69 suppresses sphingosine 1-phosophate receptor-1 (S1P1) function through interaction with membrane helix 4. J. Biol. Chem. 285, 22328–22337 (2010).
Shinoda, K. et al. Type II membrane protein CD69 regulates the formation of resting T-helper memory. Proc. Natl Acad. Sci. USA 109, 7409–7414 (2012).
Hayashizaki, K. et al. Myosin light chains 9 and 12 are functional ligands for CD69 that regulate airway inflammation. Sci. Immunol. 1, eaaf9154 (2016).
Steinert, E. M. et al. Quantifying memory CD8 T cells reveals regionalization of immunosurveillance. Cell 161, 737–749 (2015).
Siracusa, F. et al. Nonfollicular reactivation of bone marrow resident memory CD4 T cells in immune clusters of the bone marrow. Proc. Natl Acad. Sci. USA 115, 1334–1339 (2018).
Ariotti, S. et al. Tissue-resident memory CD8+ T cells continuously patrol skin epithelia to quickly recognize local antigen. Proc. Natl Acad. Sci. USA 109, 19739–19744 (2012).
Dijkgraaf, F. E. et al. Tissue patrol by resident memory CD8+ T cells in human skin. Nat. Immunol. 20, 756–764 (2019).
Sercan Alp, O. et al. Memory CD8+ T cells colocalize with IL-7+ stromal cells in bone marrow and rest in terms of proliferation and transcription. Eur. J. Immunol. 45, 975–987 (2015).
Allie, S. R. et al. The establishment of resident memory B cells in the lung requires local antigen encounter. Nat. Immunol. 20, 97–108 (2019).
Mamani-Matsuda, M. et al. The human spleen is a major reservoir for long-lived vaccinia virus-specific memory B cells. Blood 111, 4653–4659 (2008).
Martinez-Gamboa, L. et al. Role of the spleen in peripheral memory B-cell homeostasis in patients with autoimmune thrombocytopenia purpura. Clin. Immunol. 130, 199–212 (2009).
Schulz, E. G., Mariani, L., Radbruch, A. & Hofer, T. Sequential polarization and imprinting of type 1 T helper lymphocytes by interferon-γ and interleukin-12. Immunity 30, 673–683 (2009).
Richter, A., Lohning, M. & Radbruch, A. Instruction for cytokine expression in T helper lymphocytes in relation to proliferation and cell cycle progression. J. Exp. Med. 190, 1439–1450 (1999).
Lohning, M., Richter, A. & Radbruch, A. Cytokine memory of T helper lymphocytes. Adv. Immunol. 80, 115–181 (2002).
Stittrich, A. B. et al. The microRNA miR-182 is induced by IL-2 and promotes clonal expansion of activated helper T lymphocytes. Nat. Immunol. 11, 1057–1062 (2010).
Obst, R., van Santen, H. M., Mathis, D. & Benoist, C. Antigen persistence is required throughout the expansion phase of a CD4+ T cell response. J. Exp. Med. 201, 1555–1565 (2005).
Rabenstein, H. et al. Differential kinetics of antigen dependency of CD4+ and CD8+ T cells. J. Immunol. 192, 3507–3517 (2014).
Assenmacher, M. et al. Sequential production of IL-2, IFN-γ and IL-10 by individual staphylococcal enterotoxin B-activated T helper lymphocytes. Eur. J. Immunol. 28, 1534–1543 (1998).
Assenmacher, M., Schmitz, J. & Radbruch, A. Flow cytometric determination of cytokines in activated murine T helper lymphocytes: expression of interleukin-10 in interferon-γ and in interleukin-4-expressing cells. Eur. J. Immunol. 24, 1097–1101 (1994).
Lohning, M. et al. Establishment of memory for IL-10 expression in developing T helper 2 cells requires repetitive IL-4 costimulation and does not impair proliferation. Proc. Natl Acad. Sci. USA 100, 12307–12312 (2003).
Rutz, S. et al. Notch regulates IL-10 production by T helper 1 cells. Proc. Natl Acad. Sci. USA 105, 3497–3502 (2008).
McGeachy, M. J. et al. TGF-β and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain TH-17 cell–mediated pathology. Nat. Immunol. 8, 1390–1397 (2007).
Gagliani, N. et al. TH17 cells transdifferentiate into regulatory T cells during resolution of inflammation. Nature 523, 221–225 (2015).
Chang, H. D. et al. Expression of IL-10 in Th memory lymphocytes is conditional on IL-12 or IL-4, unless the IL-10 gene is imprinted by GATA-3. Eur. J. Immunol. 37, 807–817 (2007).
Peine, M. et al. Stable T-bet+GATA-3+ Th1/Th2 hybrid cells arise in vivo, can develop directly from naive precursors, and limit immunopathologic inflammation. PLoS Biol. 11, e1001633 (2013).
Hwang, E. S., Hong, J. H. & Glimcher, L. H. IL-2 production in developing Th1 cells is regulated by heterodimerization of RelA and T-bet and requires T-bet serine residue 508. J. Exp. Med. 202, 1289–1300 (2005).
Rao, D. A. et al. Pathologically expanded peripheral T helper cell subset drives B cells in rheumatoid arthritis. Nature 542, 110–114 (2017).
von Spee-Mayer, C. et al. Low-dose interleukin-2 selectively corrects regulatory T cell defects in patients with systemic lupus erythematosus. Ann. Rheum. Dis. 75, 1407–1415 (2016).
Humrich, J. Y. et al. Homeostatic imbalance of regulatory and effector T cells due to IL-2 deprivation amplifies murine lupus. Proc. Natl Acad. Sci. USA 107, 204–209 (2010).
de la Rosa, M., Rutz, S., Dorninger, H. & Scheffold, A. Interleukin-2 is essential for CD4+CD25+ regulatory T cell function. Eur. J. Immunol. 34, 2480–2488 (2004).
Black, A. P., Bhayani, H., Ryder, C. A., Gardner-Medwin, J. M. & Southwood, T. R. T-cell activation without proliferation in juvenile idiopathic arthritis. Arthritis Res. 4, 177–183 (2002).
Frenz, T. et al. CD4+ T cells in patients with chronic inflammatory rheumatic disorders show distinct levels of exhaustion. J. Allergy Clin. Immunol. 138, 586–589.e10 (2016).
Niesner, U. et al. Autoregulation of Th1-mediated inflammation by twist1. J. Exp. Med. 205, 1889–1901 (2008).
Albrecht, I. et al. Persistence of effector memory Th1 cells is regulated by Hopx. Eur. J. Immunol. 40, 2993–3006 (2010).
Alfei, F. et al. TOX reinforces the phenotype and longevity of exhausted T cells in chronic viral infection. Nature 571, 265–269 (2019).
Lee, Y. et al. Induction and molecular signature of pathogenic TH17 cells. Nat. Immunol. 13, 991–999 (2012).
Mazzoni, A. et al. Eomes controls the development of Th17-derived (non-classic) Th1 cells during chronic inflammation. Eur. J. Immunol. 49, 79–95 (2019).
Zimmermann, J. et al. T-bet expression by Th cells promotes type 1 inflammation but is dispensable for colitis. Mucosal Immunol. 9, 1487–1499 (2016).
Rebhahn, J. A. et al. An animated landscape representation of CD4+ T-cell differentiation, variability, and plasticity: insights into the behavior of populations versus cells. Eur. J. Immunol. 44, 2216–2229 (2014).
Neurath, M. F. et al. The transcription factor T-bet regulates mucosal T cell activation in experimental colitis and Crohn’s disease. J. Exp. Med. 195, 1129–1143 (2002).
Hildner, K. M. et al. Targeting of the transcription factor STAT4 by antisense phosphorothioate oligonucleotides suppresses collagen-induced arthritis. J. Immunol. 178, 3427–3436 (2007).
Miossec, P. Diseases that may benefit from manipulating the Th17 pathway. Eur. J. Immunol. 39, 667–669 (2009).
Wu, H. J. et al. Gut-residing segmented filamentous bacteria drive autoimmune arthritis via T helper 17 cells. Immunity 32, 815–827 (2010).
Dardalhon, V., Korn, T., Kuchroo, V. K. & Anderson, A. C. Role of Th1 and Th17 cells in organ-specific autoimmunity. J. Autoimmun. 31, 252–256 (2008).
Zimmermann, J. et al. The intestinal microbiota determines the colitis-inducing potential of T-bet-deficient Th cells in mice. Eur. J. Immunol. 48, 161–167 (2018).
Lexberg, M. H. et al. IFN-γ and IL-12 synergize to convert in vivo generated Th17 into Th1/Th17 cells. Eur. J. Immunol. 40, 3017–3027 (2010).
Hirota, K. et al. Fate mapping of IL-17-producing T cells in inflammatory responses. Nat. Immunol. 12, 255–263 (2011).
Bending, D. et al. Highly purified Th17 cells from BDC2.5NOD mice convert into Th1-like cells in NOD/SCID recipient mice. J. Clin. Invest. 119, 565–572 (2009).
Mazzoni, A. et al. Demethylation of the RORC2 and IL17A in human CD4+ T lymphocytes defines Th17 origin of nonclassic Th1 cells. J. Immunol. 194, 3116–3126 (2015).
Myles, A., Gearhart, P. J. & Cancro, M. P. Signals that drive T-bet expression in B cells. Cell Immunol. 321, 3–7 (2017).
Johnson, J. L., Scholz, J. L., Marshak-Rothstein, A. & Cancro, M. P. Molecular pattern recognition in peripheral B cell tolerance: lessons from age-associated B cells. Curr. Opin. Immunol. 61, 33–38 (2019).
Pham, D., Vincentz, J. W., Firulli, A. B. & Kaplan, M. H. Twist1 regulates Ifng expression in Th1 cells by interfering with Runx3 function. J. Immunol. 189, 832–840 (2012).
Haftmann, C. et al. miR-148a is upregulated by Twist1 and T-bet and promotes Th1-cell survival by regulating the proapoptotic gene Bim. Eur. J. Immunol. 45, 1192–1205 (2015).
Maschmeyer, P. et al. Selective targeting of pro-inflammatory Th1 cells by microRNA-148a-specific antagomirs in vivo. J. Autoimmun. 89, 41–52 (2018).
Hradilkova, K. et al. Regulation of fatty acid oxidation by Twist 1 in the metabolic adaptation of T helper lymphocytes to chronic inflammation. Arthritis Rheumatol. 71, 1756–1765 (2019).
Fonseka, C. Y. et al. Mixed-effects association of single cells identifies an expanded effector CD4+ T cell subset in rheumatoid arthritis. Sci. Transl. Med. 10, eaaq0305 (2018).
Zhang, F. et al. Defining inflammatory cell states in rheumatoid arthritis joint synovial tissues by integrating single-cell transcriptomics and mass cytometry. Nat. Immunol. 20, 928–942 (2019).
Arazi, A. et al. The immune cell landscape in kidneys of patients with lupus nephritis. Nat. Immunol. 20, 902–914 (2019).
Maschmeyer, P. et al. Antigen-driven PD-1+TOX+EOMES+ and PD-1+ TOX+ BHLHE40+ synovial T lymphocytes regulate chronic inflammation in situ. Eur. J. Immunol. https://doi.org/10.1002/eji.202048797 (2020).
Bardua, M. et al. MicroRNA-31 reduces the motility of proinflammatory T helper 1 lymphocytes. Front. Immunol. 9, 2813 (2018).
Petrelli, A. et al. PD-1+CD8+ T cells are clonally expanding effectors in human chronic inflammation. J. Clin. Invest. 128, 4669–4681 (2018).
Kock, J. et al. Nuclear factor of activated T cells regulates the expression of interleukin-4 in Th2 cells in an all-or-none fashion. J. Biol. Chem. 289, 26752–26761 (2014).
Podtschaske, M. et al. Digital NFATc2 activation per cell transforms graded T cell receptor activation into an all-or-none IL-2 expression. PLoS One 2, e935 (2007).
Luetke-Eversloh, M. et al. Human cytomegalovirus drives epigenetic imprinting of the IFNG locus in NKG2Chi natural killer cells. PLoS Pathog. 10, e1004441 (2014).
Babic, M. & Romagnani, C. The role of natural killer group 2, member D in chronic inflammation and autoimmunity. Front. Immunol. 9, 1219 (2018).
Gronke, K. et al. Interleukin-22 protects intestinal stem cells against genotoxic stress. Nature 566, 249–253 (2019).
Gautier, E. L. et al. Gene-expression profiles and transcriptional regulatory pathways that underlie the identity and diversity of mouse tissue macrophages. Nat. Immunol. 13, 1118–1128 (2012).
Gosselin, D. et al. Environment drives selection and function of enhancers controlling tissue-specific macrophage identities. Cell 159, 1327–1340 (2014).
Amit, I., Winter, D. R. & Jung, S. The role of the local environment and epigenetics in shaping macrophage identity and their effect on tissue homeostasis. Nat. Immunol. 17, 18–25 (2016).
Cronk, J. C. et al. Methyl-CpG binding protein 2 regulates microglia and macrophage gene expression in response to inflammatory stimuli. Immunity 42, 679–691 (2015).
Triantafyllopoulou, A. et al. Proliferative lesions and metalloproteinase activity in murine lupus nephritis mediated by type I interferons and macrophages. Proc. Natl Acad. Sci. USA 107, 3012–3017 (2010).
Arts, R. J. W., Joosten, L. A. B. & Netea, M. G. The potential role of trained immunity in autoimmune and autoinflammatory disorders. Front. Immunol. 9, 298 (2018).
Pap, T. et al. Differential expression pattern of membrane-type matrix metalloproteinases in rheumatoid arthritis. Arthritis Rheum. 43, 1226–1232 (2000).
Eljaafari, A. et al. Bone marrow-derived and synovium-derived mesenchymal cells promote Th17 cell expansion and activation through caspase 1 activation: contribution to the chronicity of rheumatoid arthritis. Arthritis Rheum. 64, 2147–2157 (2012).
Muller-Ladner, U. et al. Synovial fibroblasts of patients with rheumatoid arthritis attach to and invade normal human cartilage when engrafted into SCID mice. Am. J. Pathol. 149, 1607–1615 (1996).
Moore, J. et al. A pilot randomized trial comparing CD34-selected versus unmanipulated hemopoietic stem cell transplantation for severe, refractory rheumatoid arthritis. Arthritis Rheum. 46, 2301–2309 (2002).
Wei, K. et al. Notch signalling drives synovial fibroblast identity and arthritis pathology. Nature 582, 259–264 (2020).
Croft, A. P. et al. Distinct fibroblast subsets drive inflammation and damage in arthritis. Nature 570, 246–251 (2019).
Aungier, S. R. et al. Targeting early changes in the synovial microenvironment: a new class of immunomodulatory therapy? Ann. Rheum. Dis. 78, 186–191 (2019).
Siebert, S. et al. Targeting the rheumatoid arthritis synovial fibroblast via cyclin dependent kinase inhibition: an early phase trial. Medicine 99, e20458 (2020).
Hammer, Q. et al. Peptide-specific recognition of human cytomegalovirus strains controls adaptive natural killer cells. Nat. Immunol. 19, 453–463 (2018).
Dominguez-Andres, J., Fanucchi, S., Joosten, L. A. B., Mhlanga, M. M. & Netea, M. G. Advances in understanding molecular regulation of innate immune memory. Curr. Opin. Cell Biol. 63, 68–75 (2020).
Sekine, C. et al. Successful treatment of animal models of rheumatoid arthritis with small-molecule cyclin-dependent kinase inhibitors. J. Immunol. 180, 1954–1961 (2008).
Scheibe, F. et al. Daratumumab treatment for therapy-refractory anti-CASPR2 encephalitis. J. Neurol. 267, 317–323 (2020).
Scheibe, F. et al. Bortezomib for treatment of therapy-refractory anti-NMDA receptor encephalitis. Neurology 88, 366–370 (2017).
Kohler, S. et al. Bortezomib in antibody-mediated autoimmune diseases (TAVAB): study protocol for a unicentric, non-randomised, non-placebo controlled trial. BMJ Open 9, e024523 (2019).
Pontarini, E. et al. Treatment with belimumab restores B cell subsets and their expression of B cell activating factor receptor in patients with primary Sjogren’s syndrome. Rheumatology 54, 1429–1434 (2015).
Stohl, W. et al. Efficacy and safety of subcutaneous belimumab in systemic lupus erythematosus: a fifty-two-week randomized, double-blind, placebo-controlled study. Arthritis Rheumatol. 69, 1016–1027 (2017).
Doria, A. et al. Efficacy and safety of subcutaneous belimumab in anti-double-stranded DNA-positive, hypocomplementemic patients with systemic lupus erythematosus. Arthritis Rheumatol. 70, 1256–1264 (2018).
van Vollenhoven, R. F. et al. Long-term safety and limited organ damage in patients with systemic lupus erythematosus treated with belimumab: a Phase III study extension. Rheumatology 59, 281–291 (2020).
Zhang, F. et al. A pivotal phase III, randomised, placebo-controlled study of belimumab in patients with systemic lupus erythematosus located in China, Japan and South Korea. Ann. Rheum. Dis. 77, 355–363 (2018).
Hewett, K. et al. Randomized study of adjunctive belimumab in participants with generalized myasthenia gravis. Neurology 90, e1425–e1434 (2018).
Kraaij, T. et al. The NET-effect of combining rituximab with belimumab in severe systemic lupus erythematosus. J. Autoimmun. 91, 45–54 (2018).
Merrill, J. T. et al. Efficacy and safety of atacicept in patients with systemic lupus erythematosus: results of a twenty-four-week, multicenter, randomized, double-blind, placebo-controlled, parallel-arm, phase IIb study. Arthritis Rheumatol. 70, 266–276 (2018).
Morand, E. F. et al. Attainment of treat-to-target endpoints in SLE patients with high disease activity in the atacicept phase 2b ADDRESS II study. Rheumatology 59, 2930–2938 (2020).
He, J. et al. Low-dose interleukin-2 treatment selectively modulates CD4+ T cell subsets in patients with systemic lupus erythematosus. Nat. Med. 22, 991–993 (2016).
Acknowledgements
This work was supported by the European Research Council Advanced Grant IMMEMO (ERC-2010-AdG.20100317 Grant 268987), the Deutsche Forschungsgemeinschaft (TRR130 P16 and P15, and TRR241 B03), Innovative Medicines Initiative 2 Joint Undertaking under grant agreement no. 777357 and no. 831434, the state of Berlin and the European Regional Development Fund (ERDF 2014–2020, EFRE 1.8/11, Deutsches Rheuma-Forschungszentrum Berlin) and the Leibniz ScienceCampus Chronic Inflammation. H.D.C. is supported by the Dr. Rolf M. Schwiete Foundation.
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Rheumatology thanks V. Malmström and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Specific antigen receptors
-
Cells of the immune system express millions of different antigen receptors, each of them specifically recognizing particular structures. Each B lymphocyte expresses a particular antibody and each T lymphocyte a particular T cell receptor, enabling them to recognize and react to a defined chemical structure, the antigen.
- Immunological tolerance
-
Ablation or inactivation of B and T lymphocytes with antigen receptors specific for the body’s own and harmless antigens.
- Immunological memory
-
Imprinting and maintenance of the immune cells of an immune reaction when the reaction is over. Memory plasma cells provide protection by secretion of antibodies, and memory B and T lymphocytes provide rapid and enhanced secondary immune reactions.
- Homeostatic proliferation
-
Proliferation of memory lymphocytes induced by cytokines, in the absence of antigen.
- Plasma cell niche
-
A local environment organized by mesenchymal stromal cells, which provides survival signals for memory plasma cells.
Rights and permissions
About this article
Cite this article
Maschmeyer, P., Chang, HD., Cheng, Q. et al. Immunological memory in rheumatic inflammation — a roadblock to tolerance induction. Nat Rev Rheumatol 17, 291–305 (2021). https://doi.org/10.1038/s41584-021-00601-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41584-021-00601-6
This article is cited by
-
Warum die Regeneration von immunologischer Toleranz durch Impfen schwierig ist
Zeitschrift für Rheumatologie (2024)
-
Innate immune memory in inflammatory arthritis
Nature Reviews Rheumatology (2023)
-
Selection of cross-reactive T cells by commensal and food-derived yeasts drives cytotoxic TH1 cell responses in Crohn’s disease
Nature Medicine (2023)
-
From risk to chronicity: evolution of autoreactive B cell and antibody responses in rheumatoid arthritis
Nature Reviews Rheumatology (2022)
-
Integrated single cell and spatial transcriptomics reveal autoreactive differentiated B cells in joints of early rheumatoid arthritis
Scientific Reports (2022)