Abstract
Cardiovascular (CV) disease is the main cause of mortality in axial spondyloarthritis (axSpA). CV risk is enhanced by dysregulation of adipokines. Low omentin levels were associated with metabolic dysfunction and CV disease in conditions different from axSpA. Accordingly, we evaluated the genetic and functional implication of omentin in CV risk and subclinical atherosclerosis in a cohort of 385 axSpA patients. Subclinical atherosclerosis was evaluated by carotid ultrasound. Omentin rs12409609, in linkage disequilibrium with a polymorphism associated with CV risk, was genotyped in 385 patients and 84 controls. Serum omentin levels were also determined. omentin mRNA expression was assessed in a subgroup of individuals. Serum and mRNA omentin levels were lower in axSpA compared to controls. Low serum omentin levels were related to male sex, obesity, inflammatory bowel disease (IBD) and high atherogenic index. rs12409609 minor allele was associated with low omentin mRNA expression in axSpA. No association was observed with subclinical atherosclerosis at the genetic or functional level. In conclusion, in our study low omentin serum levels were associated with CV risk factors in axSpA. Furthermore, rs12409609 minor allele may be downregulating the expression of omentin. These data support a role of omentin as a CV risk biomarker in axSpA.
Similar content being viewed by others
Introduction
Axial spondyloarthritis (axSpA) is a chronic inflammatory disease that mainly affects the spine and pelvic joints. axSpA patients can also show a broad range of disease manifestations, including arthritis, enthesitis, dactylitis, psoriasis, uveitis and inflammatory bowel disease (IBD), among others. All this substantially affects the patient’s quality of life and has important socioeconomic consequences1.
Besides, similarly to other chronic inflammatory rheumatic diseases, axSpA is also associated with a higher incidence of hypertension, obesity, dyslipidemia and smoking habit, which are considered classic risk factors for the development of cardiovascular (CV) disease. Moreover, the inflammatory status present in those patients further enhances their CV risk2,3,4. In fact, CV disease is the main leading cause of mortality in patients with axSpA4.
In most of the cases, the first step in CV disease is the development of atherosclerosis. This process begins with damage to the vascular endothelium, triggered by the CV risk factors above mentioned, which can lead to structural changes in the vascular wall and that can be detected at the subclinical phase by non-invasive technologies such as carotid ultrasound2,5,6. In this regard, it has been shown that SpA patients develop thickening of the arterial wall and formation of carotid plaques more commonly than matched controls5,7. These morphological findings constitute surrogate markers of subclinical atherosclerosis5,7.
In this pathological context, axSpA patients usually exhibit a dysregulation of certain molecules such as adipokines, metabolic syndrome-related biomarkers and biomarkers of endothelial cell activation and inflammation, contributing thereby to the increased CV risk in axSpA8. This is why it is so important to find biomarkers that may help to predict CV risk in these patients.
Omentin (also known as intelectin 1) is a novel pleiotropic adipokine mainly secreted by visceral adipose tissue, endothelium, vascular smooth muscle, colon and small intestine, that not only exerts anti-inflammatory functions, but that has also been described as an anti-atherosclerotic and cardioprotective molecule, among other roles9. In particular, it has been demonstrated that omentin enhances insulin signal transduction and glucose uptake, attenuates vascular inflammation, has vasodilatory effect on blood vessels, suppresses reactive oxygen species production and calcification in vascular smooth muscle cells, inhibits foam cell formation and also promotes the polarization of macrophages towards the anti-inflammatory M2 phenotype9,10. Moreover, it has been reported that omentin plays a key role in bone homeostasis, inhibiting the anti-osteoblastic and pro-osteoclastic effect of activated macrophages11. Low levels of this protein have been associated with metabolic dysfunction (insulin resistance, diabetes mellitus (DM) and metabolic syndrome) and CV disease12,13,14,15. However, all these studies were performed in conditions different from axSpA such as obesity, coronary artery disease (CAD), DM and rheumatoid arthritis (RA)12,13,14,15,16.
Taking all this into consideration, in the present study we aimed to evaluate, for the first time, the genetic and functional implication of omentin in CV risk and subclinical atherosclerosis in a large cohort of axSpA.
Methods
Patients and controls
All the experiments involving humans and human blood samples were carried out in accordance with the approved guidelines and regulations, according to the Declaration of Helsinki. All experimental protocols were approved by the Ethics Committee of clinical research of Cantabria (for Hospital Universitario Marqués de Valdecilla, Santander, and Hospital Comarcal de Laredo, Laredo), Ethics Committee of clinical research of Complejo Hospitalario Universitario de Canarias (for Hospital Universitario de Canarias, Santa Cruz de Tenerife), Ethics Committee of clinical research of Madrid (for Hospital Universitario de la Princesa, Madrid), Ethics Committee of clinical research of Elda (for Hospital General Universitario de Elda, Alicante), Ethics Committee of clinical research of Ciudad Real (for Hospital General Universitario de Ciudad Real, Ciudad Real) and Ethics Committee of clinical research of Hospital Universitario de Gran Canaria Dr. Negrín (for Hospital Universitario de Gran Canaria Dr. Negrín, Las Palmas de Gran Canaria). Informed written consent was obtained from all subjects.
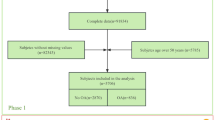
385 Spanish patients who fulfilled the Assessment of SpondyloArthritis international Society (ASAS) classification criteria for axSpA17 were recruited for this study at Hospital Universitario Marqués de Valdecilla (Santander), Hospital Comarcal de Laredo (Laredo), Hospital Universitario de Canarias (Santa Cruz de Tenerife), Hospital Universitario de La Princesa (Madrid), Hospital General Universitario de Elda (Alicante), Hospital General Universitario de Ciudad Real (Ciudad Real) and Hospital Universitario de Gran Canaria Dr. Negrín (Las Palmas de Gran Canaria). None of them had DM or chronic kidney disease. 84 Spanish healthy controls (who did not have history of CV events or chronic inflammatory diseases) were also recruited for the comparative analysis of omentin (at the genetic and functional level) between axSpA patients and controls.
Data on sex, age, body mass index (BMI), blood pressure, total cholesterol, high-density lipoprotein (HDL)- and low-density lipoprotein (LDL)-cholesterol and triglycerides at the time of study, as well as history of traditional CV risk factors (smoking, obesity, dyslipidemia and hypertension) were collected. Obesity was defined if BMI (calculated as weight in kilograms divided by height in squared meters) was ≥30. Individuals were considered to have dyslipidemia if they had hypercholesterolemia and/or hypertriglyceridemia (defined as diagnosis of hypercholesterolemia or hypertriglyceridemia by the individuals’ family physician, or total cholesterol and/or triglyceride levels in fasting plasma being>220 and>150 mg/dL, respectively). In those individuals with total cholesterol between 200 and 220 mg/dL, a diagnosis of dyslipidemia was considered if the atherogenic index (AI) (total cholesterol/HDL-cholesterol) was ≥4. Individuals were diagnosed as having hypertension if blood pressure was>140/90 mmHg or if they were taking antihypertensive agents. Routine laboratory parameters such as C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR) were assessed at the time of the study. The main demographic, clinical, laboratory and CV disease-related data of patients are displayed in Table 1.
Peripheral blood samples were collected in the fasting state from all the patients and controls at the time of recruitment.
Carotid ultrasound study
A carotid ultrasound study was performed in all the patients to assess the presence of abnormal carotid intima-media thickness (cIMT) values in the common carotid artery as well as the presence of focal plaques in the extracranial carotid tree (as surrogate markers of subclinical atherosclerosis), as previously reported7.
Omentin genotyping
DNA of patients and controls was obtained from peripheral blood using NucleoSpin Blood Kit (Macherey-Nagel, Germany), according to the manufacturer’s instructions. The omentin rs12409609 (C/T) polymorphism, in complete linkage disequilibrium with rs2274907 (previously associated with CV risk18,19,20), was genotyped by a pre-designed TaqMan SNP genotyping assay (C___1757143_10) in a 7900 HT real-time instrument (Applied Biosystems, USA), according to the conditions recommended by the manufacturer.
Serum omentin assay
A commercial enzyme-linked immunosorbent assay (ELISA) kit was used to measure serum omentin levels in axSpA patients and controls (RD191100200R, BioVendor, Czech Republic) according to the manufacturer’s instructions. All samples were analyzed in duplicate and quantified relative to a standard curve, using a 4-parameter algorithm.
Omentin mRNA expression analysis
RNA was extracted from peripheral blood samples of 176 axSpA patients and 27 controls using the NucleoSpin RNA Blood Kit (Macherey-Nagel, Germany), according to the manufacturer’s instructions. Total RNA was reverse transcribed into complementary DNA (cDNA) using iScriptTM Advanced cDNA Synthesis Kit for reverse transcription- quantitative real-time PCR (qPCR) (Bio-Rad, USA), and cDNA was amplified with SsoAdvancedTM Universal SYBR® Green Supermix (Bio-Rad, USA). qPCR reactions for omentin (target gene) and GAPDH (housekeeping gene) were performed in custom 384-well-plates (PrimePCR Assays, Bio-Rad, USA) in a 7900 HT real-time instrument (Applied Biosystems, USA). All samples were assayed in duplicate and experimental control assays were included. The relative omentin mRNA expression was analyzed by the comparative Ct method, as previously reported21. Normalized values were obtained for each sample and mean values were determined for each study group.
Statistical analysis
The omentin rs12409609 genotype data were checked for deviation from Hardy-Weinberg equilibrium (HWE). Differences in the distribution of genotypes and alleles between patients and controls were assessed by chi-squared test. Strength of associations were estimated using odds ratios (OR) and 95% confidence intervals (CI). The relationship between genotypes or alleles and carotid plaques was tested using logistic regression, while the association with cIMT values was evaluated by ANOVA, in both cases adjusting for potential confounding factors: sex, age at the time of the study and classic CV risk factors (smoking, obesity, dyslipidemia and hypertension). The link between genotypes or alleles with serum levels or mRNA expression of omentin was tested by linear regression, adjusting for potential confounding factors. In all the genetic analyses, the most frequent genotype and allele of omentin rs12409609 (CC and C, respectively) was used as reference.
The Shapiro-Wilk normality test was performed and showed that serum levels and normalized mRNA expression of omentin were not normally distributed in our cohorts. Accordingly, these data were log transformed for the statistical analysis. Differences in serum levels or mRNA expression of omentin between patients and controls were assessed by linear regression. The association of serum levels or mRNA expression of omentin with carotid plaques was tested by logistic regression, while the correlation between serum levels or mRNA expression of omentin and cIMT values was performed via estimation of the Pearson partial correlation coefficient (r). The association of serum levels of omentin with categorical and continuous variables was assessed by linear regression and Pearson’s partial correlation coefficient (r), respectively. In all the cases, adjustment was performed for the potential confounding factors above mentioned.
Data were expressed as mean ± standard deviation (SD) for continuous variables, and number of individuals (n) or percentage (%) for categorical variables. Statistical significance was defined as p values ≤0.05, and all analyses were performed using STATA® v. 11.1 statistical software (Stata Corp, College Station, TX, USA).
Results
Genotype and allele distribution of omentin rs12409609
Genotyping success was greater than 98%. Omentin rs12409609 genotype distribution was in HWE (p > 0.05). Genotype and allele frequencies of rs12409609 were in agreement with the data of the 1000 Genomes Project for Europeans. In addition, the distribution of the genotypes and alleles of rs12409609 was similar between axSpA patients and controls (p > 0.05) (Table 2).
Differences in serum and mRNA expression levels of omentin
Serum omentin levels were significantly lower in patients than in controls (361.6 ± 129.6 vs. 449.5 ± 154.2 ng/mL, respectively, p < 0.001) (Fig. 1a). Interestingly, serum omentin levels were higher in females when compared to males (389.6 ± 150.8 vs. 346.0 ± 113.5 ng/mL, in female and male patients, respectively, p = 0.013). Furthermore, the mRNA expression of omentin was also nearly 3-fold lower in patients when compared to controls (30.5 ± 29.6 vs. 76.0 ± 61.0, respectively, p < 0.001) (Fig. 1b).
Omentin levels in axSpA and controls. Reduced serum (a) and mRNA expression (b) levels of omentin in axSpA patients when compared to healthy controls, after adjustment for potential confounding factors. Horizontal bars in Fig. 1a indicate the mean value of each group.
Relationship of omentin with clinical features and CV risk factors
We disclosed that low serum omentin levels were associated with obesity and AI indicative of dyslipidemia (AI ≥ 4) in axSpA patients (317.1 ± 126.8 ng/mL in obese vs. 372.3 ± 128.4 ng/mL in non-obese patients, p < 0.001, Fig. 2a; 329.5 ± 106.1 ng/mL in patients with AI ≥ 4 vs. 389.6 ± 136.9 ng/mL in patients with AI < 4, p = 0.006, Fig. 2b). In addition, we observed that patients with IBD also exhibited lower serum levels of omentin (295.2 ± 76.1 vs. 367.7 ± 132.0 ng/mL in patients with and without IBD, respectively, p = 0.019) (Fig. 2c).
Omentin serum levels and cardiovascular risk factors. Differences in serum omentin levels between obese and non-obese axSpA patients (a), axSpA patients showing or not an AI indicative of dyslipidemia (AI ≥ 4) (b), and axSpA patients with and without IBD (c), after adjustment for potential confounding factors. Horizontal bars indicate the mean value of each group.
Influence of rs12409609 on serum levels and mRNA expression levels of omentin
We found that patients homozygous for the minor allele of rs12409609 (TT genotype) showed the lowest mRNA expression level when compared to those carrying the CC genotype (9.9 ± 6.4 vs. 38.4 ± 35.7, respectively, p < 0.001), while patients bearing the CT genotype displayed intermediate mRNA expression levels (25.3 ± 21.4, p = 0.008) (Fig. 3). Likewise, it was disclosed that the T allele was associated with low gene expression of omentin in our patients (21.4 ± 20.0 in patients bearing the T allele vs. 34.2 ± 32.2 in those carrying the C allele, p < 0.001) (Fig. 3). No statistically significant association was observed between rs12409609 alleles and genotypes and serum levels of omentin (p > 0.05).
Association of omentin and surrogate markers of subclinical atherosclerosis
No statistically significant association was observed between omentin and markers of subclinical atherosclerosis (presence of carotid plaques and abnormal cIMT values) at the genetic or functional (protein and mRNA) level (p > 0.05).
Discussion
CV disease is the leading cause of mortality in axSpA patients4. Accordingly, it is of main clinical relevance to find non-invasive blood biomarkers that, along with clinical features and imaging techniques, could help us to identify axSpA patients at risk of CV events. For this purpose, in this study we aimed to assess the implication of omentin, a novel adipokine, in the CV risk and subclinical atherosclerosis of axSpA patients. In the last years this molecule has attracted an increasing interest and, consequently, several studies were performed to evaluate the role of omentin in diseases different from axSpA12,13,14,15,16. However, despite the clinical importance of the CV risk in axSpA, to the best of our knowledge, this is the first study aimed to address this issue in this chronic inflammatory disease.
In the present study we found that serum levels of omentin were reduced in axSpA patients when compared to controls. This is in line with previous studies performed in DM, CAD and psoriasis, in which decreased levels of omentin were described in such conditions13,22,23,24,25,26,27. Additionally, we noted that the serum levels of omentin were higher in women than in men. This finding on the sexual dimorphism of omentin had been previously described, and was associated with the different pattern of body fat distribution in women and men, as well as with a potential influence of sex hormones (such as testosterone) on the regulation of omentin28.
In relation to the mRNA expression of omentin, the studies performed so far were mainly focused on assessing the potential difference in its expression in adipose tissue between several diseases and healthy controls. With respect to this, it has been described that low omentin expression levels were observed in patients with inflammatory conditions such as CAD, obesity or polycystic ovary syndrome12,13,29. In keeping with these observations, in our study we disclosed a decreased mRNA expression of omentin in whole blood in axSpA when compared to controls, similar to the results we obtained at the serum level.
As for its anti-atherogenic function, similar to the results obtained in RA by Robinson et al.16, in our study we did not find any direct association between omentin and surrogate markers of subclinical atherosclerosis (presence of carotid plaques and abnormal cIMT values) in axSpA. Notwithstanding, an association between low serum levels of omentin and CV risk factors such as obesity and AI indicative of dyslipidemia was observed in our axSpA patients. This is in accordance with previous reports from studies performed in the general population and in DM patients, in which a negative correlation between omentin levels and obesity was described12,30,31,32,33. In this regard, it was proposed that molecules produced as a result of obesity-induced chronic low-grade inflammation may be modulating omentin levels10,12. Likewise, in line with our results, other authors reported an inverse association of omentin levels with adverse lipid profiles in conditions different from axSpA12,31,33. High AI constitutes a CV risk factor that contributes to the development of atherosclerosis34.
In addition, we also disclosed low serum levels of omentin in patients with IBD. This is in agreement with previous reports that showed that serum omentin is decreased in patients with active Crohn’s disease and ulcerative colitis, two types of IBD35,36. Accordingly, serum omentin has been suggested as a potential marker of IBD disease activity10,35,36. Interestingly, in line with these results, the expression of omentin in the colonic tissue of patients with active Crohn’s disease was also reported to be downregulated35. All these data suggest that the lower levels of omentin observed in IBD patients reflect their inflammatory state, both systemically and locally, supporting its anti-inflammatory function. Importantly, recent studies indicate that IBD could be associated with a higher CV risk37. This may be due to the oxidative stress and high circulating levels of pro-inflammatory molecules that are the result of the systemic inflammation observed in IBD patients, and that might contribute to the development of endothelial damage and atherosclerosis37,38.
At the genetic level, we genotyped rs12409609, a genetic variant located in the downstream regulatory region of omentin and that is in complete linkage disequilibrium with rs2274907, a missense polymorphism of the gene previously associated with CV risk18,19,20. In our study we did not observe any relationship between rs12409609 and axSpA nor with subclinical atherosclerosis in these patients. Similar results were obtained in inflammatory conditions such as psoriasis, DM or rheumatoid arthritis23,39,40. Interestingly, however, we noted that the minor allele of omentin rs12409609 was linked to a reduced mRNA expression of omentin, being this association dose-dependent. In this regard, patients bearing a single or double dose of the minor allele (T) showed intermediate or the lowest omentin expression levels, respectively, when compared to those patients homozygous for the most frequent allele (C). Nevertheless, further replication studies are needed to confirm the influence of this polymorphism on omentin mRNA expression.
Based on our data and on those reported in previous studies, it seems clear that changes in omentin levels are implicated in the pathology of conditions related to inflammation and cardiovascular disease, given its pleiotropic functions. However, the cross-sectional design of our study precludes drawing conclusions about whether the lower levels of omentin observed in our axSpA patients are the cause or the consequence of their high CV risk. In fact, the results from two prospective studies performed so far on the predictive role of omentin in CV disease are controversial. One of them proposes that low levels of omentin predict the outcome of adverse cardiac events in patients with hypertrophic cardiomyopathy41, while the other reports that high omentin predicts CV events in coronary patients42, possibly as a compensatory anti-inflammatory and cardioprotective mechanism. Therefore, further longitudinal studies in this regard are warranted.
In conclusion, our data provide evidence for the first time that omentin is linked to obesity and adverse lipid profiles in axSpA. Additionally, low serum levels of this protein are associated with the presence of IBD in axSpA. Furthermore, our results suggest that the minor allele of omentin rs12409609 may be downregulating the expression of this gene in whole blood of axSpA patients. Taking all this into consideration, our data support the role of omentin as a CV risk biomarker in axSpA. Accordingly, omentin may constitute a promising target for future therapeutic strategies aimed to increase its circulating levels and, thereby, prevent the development of CV disease in axSpA patients. This would not only lead to a beneficial effect on CV risk but also on features typical of axSpA such as inflammation and bone remodeling, further supporting the relevance of this molecule at the clinical level.
Data availability
All data generated or analysed during this study are included in this published article.
References
Raine, C. & Keat, A. Axial spondyloarthritis. Medicine. 46, 231–236 (2018).
Papagoras, C., Voulgari, P. V. & Drosos, A. A. Atherosclerosis and cardiovascular disease in the spondyloarthritides, particularly ankylosing spondylitis and psoriatic arthritis. Clin. Exp. Rheumatol. 31, 612–620 (2013).
Papagoras, C. et al. Cardiovascular risk profile in patients with spondyloarthritis. Joint Bone Spine. 81, 57–63 (2014).
Moltó, A. & Nikiphorou, E. Comorbidities in Spondyloarthritis. Front. Med. (Lausanne). 5, 62, https://doi.org/10.3389/fmed.2018.00062 (2018).
Gonzalez-Juanatey, C. et al. The high prevalence of subclinical atherosclerosis in patients with ankylosing spondylitis without clinically evident cardiovascular disease. Medicine (Baltimore). 88, 358–365 (2009).
Castañeda, S., Nurmohamed, M. T. & González-Gay, M. A. Cardiovascular disease in inflammatory rheumatic diseases. Best. Pract. Res. Clin. Rheumatol. 30, 851–869 (2016).
Rueda-Gotor, J. et al. Atherosclerotic disease in axial spondyloarthritis: increased frequency of carotid plaques. Clin. Exp. Rheumatol. 33, 315–320 (2015).
Genre, F. et al. Adipokines, biomarkers of endothelial activation, and metabolic syndrome in patients with ankylosing spondylitis. Biomed. Res. Int. 2014, 860651, https://doi.org/10.1155/2014/860651 (2014).
Watanabe, T., Watanabe-Kominato, K., Takahashi, Y., Kojima, M. & Watanabe, R. Adipose Tissue-Derived Omentin-1 Function and Regulation. Compr. Physiol. 7, 765–781 (2017).
Tan, Y. L., Zheng, X. L. & Tang, C. K. The protective functions of omentin in cardiovascular diseases. Clin. Chim. Acta. 448, 98–106 (2015).
Rao, S. S. et al. Omentin-1 prevents inflammation-induced osteoporosis by downregulating the pro-inflammatory cytokines. Bone. Res. 6, 9, https://doi.org/10.1038/s41413-018-0012-0 (2018).
de Souza Batista, C. M. et al. Omentin plasma levels and gene expression are decreased in obesity. Diabetes. 56, 1655–1661 (2007).
Du, Y. et al. Association between omentin-1 expression in human epicardial adipose tissue and coronary atherosclerosis. Cardiovasc. Diabetol. 15, 90, https://doi.org/10.1186/s12933-016-0406-5 (2016).
Shibata, R. et al. Omentin as a novel biomarker of metabolic risk factors. Diabetol. Metab. Syndr. 4, 37, https://doi.org/10.1186/1758-5996-4-37 (2012).
Tan, B. K. et al. Decreased plasma omentin-1 levels in Type 1 diabetes mellitus. Diabet. Med. 25, 1254–1255 (2008).
Robinson, C. et al. Omentin concentrations are independently associated with those of matrix metalloproteinase-3 in patients with mild but not severe rheumatoid arthritis. Rheumatol. Int. 37, 3–11 (2017).
Sieper, J. et al. The Assessment of SpondyloArthritis international Society (ASAS) handbook: a guide to assess spondyloarthritis. Ann. Rheum. Dis. 68, ii1–44 (2009).
Jamshidi, J., Ghanbari, M., Asnaashari, A., Jafari, N. & Valizadeh, G. A. Omentin Val109Asp polymorphism and risk of coronary artery disease. Asian Cardiovasc. Thorac. Ann. 25, 199–203 (2017).
Nazar, S., Zehra, S. & Azhar, A. Association of single Nucleotide Missence Polymorphism Val109Asp of Omentin-1 gene and coronary artery disease in Pakistani population: Multicenter study. Pak. J. Med. Sci. 33, 1128–1133 (2017).
Khoshi, A., Bajestani, M. K., Shakeri, H., Goodarzi, G. & Azizi, F. Association of Omentin rs2274907 and FTO rs9939609 gene polymorphisms with insulin resistance in Iranian individuals with newly diagnosed type 2 diabetes. Lipids. Health. Dis. 18, 142, https://doi.org/10.1186/s12944-019-1085-5 (2019).
Remuzgo-Martínez, S. et al. Decreased expression of methylene tetrahydrofolate reductase (MTHFR) gene in patients with rheumatoid arthritis. Clin. Exp. Rheumatol. 34, 106–110 (2016).
Smékal, A. et al. Plasma levels and leucocyte RNA expression of adipokines in young patients with coronary artery disease, in metabolic syndrome and healthy controls. Cytokine. 122, 154017, https://doi.org/10.1016/j.cyto.2017.03.016 (2019).
Turan, H. et al. Omentin serum levels and omentin gene Val109Asp polymorphism in patients with psoriasis. Int. J. Dermatol. 53, 601–605 (2014).
Elsaid, N. H., Sadik, N. A., Ahmed, N. R., Fayez, S. E. & Mohammed, N. A. E. Serum omentin-1 levels in type 2 diabetic obese women in relation to glycemic control, insulin resistance and metabolic parameters. J. Clin. Transl. Endocrinol. 13, 14–19 (2018).
Greulich, S. et al. Cardioprotective properties of omentin-1 in type 2 diabetes: evidence from clinical and in vitro studies. PLoS One. 8, e59697, https://doi.org/10.1371/journal.pone.0059697 (2013).
Motawi, T. M. K., Mahdy, S. G., El-Sawalhi, M. M., Ali, E. N. & El-Telbany, R. F. A. Serum levels of chemerin, apelin, vaspin, and omentin-1 in obese type 2 diabetic Egyptian patients with coronary artery stenosis. Can. J. Physiol. Pharmacol. 96, 38–44 (2018).
Zhang, C. et al. Omentin-1 plasma levels and omentin-1 expression are decreased in psoriatic lesions of psoriasis patients. Arch. Dermatol. Res. 307, 455–459 (2015).
Luque-Ramírez, M. et al. Sexual dimorphism in adipose tissue function as evidenced by circulating adipokine concentrations in the fasting state and after an oral glucose challenge. Hum. Reprod. 28, 1908–1918 (2013).
Tan, B. K. et al. Omentin-1, a novel adipokine, is decreased in overweight insulin-resistant women with polycystic ovary syndrome: ex vivo and in vivo regulation of omentin-1 by insulin and glucose. Diabetes. 57, 801–808 (2008).
Sitticharoon, C. et al. Interactions between adiponectin, visfatin, and omentin in subcutaneous and visceral adipose tissues and serum, and correlations with clinical and peripheral metabolic factors. Peptides. 62, 164–175 (2014).
Zhang, Q. et al. Changes of serum omentin-1 levels in normal subjects, type 2 diabetes and type 2 diabetes with overweight and obesity in Chinese adults. Ann. Endocrinol. (Paris). 75, 171–175 (2014).
Yan, P. et al. Changes of serum omentin levels and relationship between omentin and adiponectin concentrations in type 2 diabetes mellitus. Exp. Clin. Endocrinol. Diabetes. 119, 257–263 (2011).
Vu, A., Sidhom, M. S., Bredbeck, B. C., Kosmiski, L. A. & Aquilante, C. L. Evaluation of the relationship between circulating omentin-1 concentrations and components of the metabolic syndrome in adults without type 2 diabetes or cardiovascular disease. Diabetol. Metab. Syndr. 6, 4, https://doi.org/10.1186/1758-5996-6-4 (2014).
González-Gay, M. A. & Rueda-Gotor, J. Atherosclerosis in ankylosing spondylitis: clinical implications. Pol. Arch. Intern. Med. 128, 409–410 (2018).
Lu, Y. et al. Serum omentin-1 as a disease activity marker for Crohn’s disease. Dis. Markers. 2014, 162517, https://doi.org/10.1155/2014/162517 (2014).
Yin, J., Hou, P., Wu, Z. & Nie, Y. Decreased Levels of Serum omentin-1 in Patients With Inflammatory Bowel Disease. Med Sci Monit. 21, 118–122 (2015).
Schicho, R., Marsche, G. & Storr, M. Cardiovascular complications in inflammatory bowel disease. Curr. Drug. Targets. 16, 181–188 (2015).
Wu, P., Jia, F., Zhang, B. & Zhang, P. Risk of cardiovascular disease in inflammatory bowel disease. Exp. Ther. Med. 13, 395–400 (2017).
Schäffler, A. et al. Frequency and significance of the novel single nucleotide missense polymorphism Val109Asp in the human gene encoding omentin in Caucasian patients with type 2 diabetes mellitus or chronic inflammatory bowel diseases. Cardiovasc. Diabetol. 6, 3, https://doi.org/10.1186/1475-2840-6-3 (2007).
Yaykasli, K. O. & Yaykasli, E. The frequency of omentin Val109Asp polymorphism and the serum level of omentin in patients with rheumatoid arthritis. Acta Medica Mediterranea. 29, 52 (2013).
Yıldız, S. S. et al. Usefulness of Serum Omentin-1 Levels for the Prediction of Adverse Cardiac Events in Patients with Hypertrophic Cardiomyopathy. Med Princ Pract. 27, 107–114 (2018).
Saely, C. H. et al. High plasma omentin predicts cardiovascular events independently from the presence and extent of angiographically determined atherosclerosis. Atherosclerosis. 244, 38–43 (2016).
Acknowledgements
We wish to thank all the patients and controls that participated in this study. This work was supported by funds of a NEXT-VAL grant (NVAL17/10) (Instituto de Investigación Sanitaria IDIVAL) awarded to FG. SR-M is supported by funds of the RETICS Program (RD16/0012/0009) from the ‘Instituto de Salud Carlos III´ (ISCIII), co-funded by the European Regional Development Fund (ERDF). VP-C is supported by a pre-doctoral grant from IDIVAL (PREVAL 18/01). VM is supported by funds of a Miguel Servet type I programme (grant CP16/00033) (ISCIII, co-funded by the European Social Fund (ESF)). LL-G is supported by funds of PI18/00042 (ISCIII, co-funded by ERDF). RL-M is a recipient of a Miguel Servet type I programme fellowship from the ISCIII, co-funded by the ESF (grant CP16/00033).
Author information
Authors and Affiliations
Contributions
F.G., J.R.-G. and S.R.-M. carried out the conception and design of the study, were involved in the statistical analysis and interpretation of data, and in the elaboration of the manuscript. V.P.-.C., A.C., V.M., L.L.-G., V.P., R.E., C.M., R.B., J.L., V.H.-.H., E.V., C.F.-C., M.P.M.-V., D.C.-C., J.A.-F., C.R.-L., O.G., J.C.Q.-A., S.C. and I.F.-A helped in the acquisition and interpretation of data, and contributed to the elaboration of the manuscript. RL-M and MAG-G contributed to the elaboration of the protocol of study, helped in the interpretation of data and were responsible of the final drafting and elaboration of the manuscript. All authors have approved the final article.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Genre, F., Rueda-Gotor, J., Remuzgo-Martínez, S. et al. Omentin: a biomarker of cardiovascular risk in individuals with axial spondyloarthritis. Sci Rep 10, 9636 (2020). https://doi.org/10.1038/s41598-020-66816-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-66816-x
This article is cited by
-
Cardiovascular risk in axial spondyloarthritis—a systematic review
Clinical Rheumatology (2023)
-
Biomarkers in axial spondyloarthritis and low back pain: a comprehensive review
Clinical Rheumatology (2022)
-
Factors associated with high cardiovascular risk in psoriatic arthritis and non-psoriatic spondyloarthritis
Rheumatology International (2022)
-
Vaspin in atherosclerotic disease and cardiovascular risk in axial spondyloarthritis: a genetic and serological study
Arthritis Research & Therapy (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.