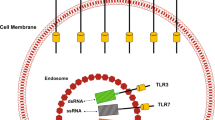
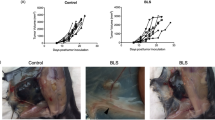
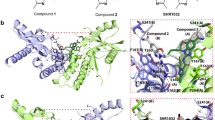
Abstract
Small-molecule agonists at Toll-like receptor 7 (TLR7) and TLR8 have sparked a vivid interest in cancer research owing to their profound antitumoral activity. The lead compound of the imidazoquinoline family, imiquimod, is marketed as a topical formulation. It is efficacious against many primary skin tumors and cutaneous metastases. Using different imidazoquinoline species, distinct functions of TLR7 and TLR8 have been discovered. The predominant antitumoral mode of action of these agents is TLR7/8-mediated activation of the central transcription factor nuclear factor-κB, which leads to induction of proinflammatory cytokines and other mediators. Cutaneous dendritic cells are the primary responsive cell type and initiate a strong Th1-weighted antitumoral cellular immune response. Recent research has shown that dendritic cells themselves acquire direct antitumoral activity upon stimulation by imiquimod. In addition, there are a number of secondary effects on the molecular and cellular level that can be explained through the activation of TLR7/8. The proinflammatory activity of imiquimod, but not resiquimod, appears to be augmented by suppression of a regulatory mechanism, which normally limits inflammatory responses. This is achieved independently of TLR7/8 through interference with adenosine receptor signaling pathways. Finally, at higher concentrations imiquimod exerts Bcl-2- and caspase-dependent proapoptotic activity against tumor cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Ambach A, Bonnekoh B, Nguyen M, Schön MP, Gollnick H . (2004). Imiquimod, a toll-like receptor-7 agonist, induces perforin in cytotoxic T lymphocytes in vitro. Mol Immunol 40: 1307–1314.
Barland CO, Zettersten E, Brown BS, Ye J, Elias PM, Ghadially R . (2004). Imiquimod-induced interleukin-1 alpha stimulation improves barrier homeostasis in aged murine epidermis. J Invest Dermatol 122: 330–336.
Bath-Hextall F, Bong F, Perkins W, Williams H . (2004). Interventions for basal cell carcinoma of the skin: systematic review. Br Med J 329: 705.
Berman B, Sullivan TP, De Araujo T, Nadji T . (2003). Expression of Fas-receptor on basal cell carcinomas after treatment with imiquimod 5% cream or vehicle. Br J Dermatol 149 (Suppl. 66): 59–61.
Bernstein DI, Harrison CJ, Tepe ER, Shahwan A, Miller RL . (1995). Effect of imiquimod as an adjuvant for immunotherapy of genital HSV in guinea pigs. Vaccine 13: 72–76.
Bernstein DI, Harrison CJ, Tomai MA, Miller RL . (2000). Daily or weekly therapy with resiquimod (R848) reduces genital recurrences in herpes simplex virus-infected guinea pigs during and after treatment. J Infect Dis 183: 844–849.
Bishop GA, Ramirez LM, Baccam M, Busch LK, Pederson LK, Tomai MA . (2001). The immune response modifier resiquimod mimics CD40-induced B cell activation. Cell Immunol 25: 9–17.
Bong AB, Bonnekoh B, Franke I, Schön MP, Ulrich J, Gollnick H . (2002). Imiquimod, a novel immune response modifier, in the treatment of cutaneous metastases of malignant melanoma. Dermatology 205: 135–138.
Bottrell RL, Yang YL, Levy DE, Tomai MA, Reis LF . (1999). The immune response modifier imiquimod requires STAT-1 for induction of interferon, interferon-stimulated genes, and interleukin-6. Antimicrob Agents Chemother 43: 856–861.
Brown VL, Atkins CL, Ghali L, Cerio R, Harwood CA, Proby CM . (2005). Safety and efficacy of 5% imiquimod cream for the treatment of skin dysplasia in high-risk renal transplant recipients: randomized, double-blind, placebo-controlled trial. Arch Dermatol 141: 985–993.
Buates S, Matlashewski G . (2001). Identification of genes induced by a macrophage activator, S-28463, using gene expression array analysis. Antimicrob Agents Chemother 45: 1137–1142.
Burns R, Ferbel B, Tomai MA, Miller RL, Gaspari A . (2000). The imidazoquinolines, imiquimod and R-848, induce functional, but not phenotypic, maturation of human epidermal Langerhans cells. Clin Immunol 94: 13–23.
Chen M, Griffith BP, Lucia HL, Hsiung GD . (1988). Efficacy of S-26308 against guinea pig cytomegalovirus infection. Antimicrob Agents Chemother 32: 678–683.
Chong A, Loo WJ, Banney L, Grant JW, Norris PG . (2004). Imiquimod 5% cream in the treatment of mycosis fungoides—a pilot study. J Dermatolog Treat 15: 118–119.
Craft N, Bruhn KW, Nguyen BD, Prins RM, Lin JW, Liau LM et al. (2005). The TLR7 agonist imiquimod enhances the anti-melanoma effects of a recombinant Listeria monocytogenes vaccine. J Immunol 175: 1983–1990.
Deeths MJ, Chapman JT, Dellavalle RP, Zeng C, Aeling JL . (2005). Treatment of patch and plaque stage mycosis fungoides with imiquimod 5% cream. J Am Acad Dermatol 52: 275–280.
Dendorfer M, Oppel T, Wollenberg A, Prinz JC . (2003). Topical treatment with imiquimod may induce regression of facial keratoacanthoma. Eur J Dermatol 13: 80–82.
Diebold SS, Kaisho T, Hemmi H, Akira S, Reis e Sousa C . (2004). Innate antiviral responses by means of single-stranded RNA. Science 303: 1529–1531.
Dockrell DH, Kinghorn GR . (2001). Imiquimod and resiquimod as novel immunomodulators. J Antimicrob Chemother 48: 751–755.
Dummer R, Urosevic M, Kempf W, Hoek K, Hafner J, Burg G . (2003a). Imiquimod in basal cell carcinoma: how does it work? Br J Dermatol 149 (Suppl. 66): 57–58.
Dummer R, Urosevic M, Kempf W, Kazakov D, Burg G . (2003b). Imiquimod induces complete clearance of a PUVA-resistant plaque in mycosis fungoides. Dermatology 207: 116–118.
Fleming CJ, Bryden AM, Evans A, Dawe RS, Ibbotson SH . (2004). A pilot study of treatment of lentigo maligna with 5% imiquimod cream. Br J Dermatol 151: 485–488.
Frotscher B, Anton K, Worm M . (2002). Inhibition of IgE production by the imidazoquinoline resiquimod in nonallergic and allergic donors. J Invest Dermatol 119: 1059–1064.
Geisse J, Caro I, Lindholm J, Golitz L, Stampone P, Owens M . (2004). Imiquimod 5% cream for the treatment of superficial basal cell carcinoma: results from two phase III, randomized, vehicle-controlled studies. J Am Acad Dermatol 50: 722–733.
Gerster JF, Lindstrom KJ, Miller RL, Tomai MA, Birmachu W, Bomersine SN et al. (2005). Synthesis and structure-activity-relationships of 1H-imidazo[4,5-c]quinolines that induce interferon production. J Med Chem 48: 3481–3491.
Giannotti B, Vanzi L, Difonzo EM, Pimpinelli N . (2003). The treatment of basal cell carcinomas in a patient with Xeroderma pigmentosum with a combination of imiquimod 5% cream and oral acitretin. Clin Exp Dermatol 28 (Suppl. 1): 33–35.
Gibson SJ, Imbertson LM, Wagner TL . (1995). Cellular requirements for cytokine production in response to the immunomodulators imiquimod and S-27609. J Interferon Cytokine Res 15: 537–545.
Gibson SJ, Lindh JM, Riter TR, Gleason RM, Rogers LM, Fuller AE et al. (2002). Plasmacytoid dendritic cells produce cytokines and mature in response to the TLR7 agonists, imiquimod and resiquimod. Cell Immunol 218: 74–86.
Gollnick H, Barasso R, Jappe U, Ward K, Eul A, Carey-Yard M et al. (2001). Safety and efficacy of imiquimod 5% cream in the treatment of penile genital warts in uncircumcised men when applied three times weekly or one per day. Int J STD AIDS 12: 22–28.
Gollnick H, Barona CG, Frank RG, Ruzicka T, Megahed M, Tebbs V et al. (2005). Recurrence rate of superficial basal cell carcinoma following successful treatment with imiquimod 5% cream: interim 2-year results from an ongoing 5-year follow-up study in Europe. Eur J Dermatol 15: 374–381.
Gorden KB, Gorski KS, Gibson SJ, Kedl RM, Kieper WC, Qiu X et al. (2005). Synthetic TLR agonists reveal functional differences between human TLR7 and TLR8. J Immunol 174: 1259–1268.
Gorden KB, Qiu X, Binsfeld CCA, Vasilakos JP, Alkan SS . 2006a. Cutting edge: Activation of murine TLR8 by a combination of imidazoquinoline immune response modifiers and polyT oligodeoxynucleotides. J Immunol 177: 6584–6587.
Gorden KB, Xiaohong Q, Battiste JJL, Wightman PPD, Vasilakos JP, Alkan SS . (2006b). Oligodeoxynucleotides differentially modulate activation of TLR7 and TLR8 by imidazoquinolines. J Immunol 177: 8164–8170.
Gorski KS, Waller EL, Bjornton-Severson J, Hanten JA, Riter CL, Kieper WC et al. (2006). Distinct indirect pathways govern human NK-cell activation by TLR-7 and TLR-8 agonists. Int Immunol 18: 1115–1126.
Hadley G, Derry S, Moore RA . 2006. Imiquimod for actinic keratosis: systematic review and meta-analysis. J Invest Dermatol 126: 1251–1255.
Harrison CJ, Jenski L, Voychehovski T, Bernstein DI . (1988). Modification of immunological responses and clinical disease during topical R-837 treatment of genital HSV-2 infection. Antiviral Res 10: 209–224.
Harrison CJ, Miller RL, Bernstein DI . (1994). Posttherapy suppression of genital herpes simplex virus (HSV) recurrences and enhancement of HSV-specific T-cell memory by imiquimod in guinea pigs. Antimicrob Agents Chemother 38: 2059–2064.
Heil F, Ahmad-Nejad P, Hemmi H, Hochrein H, Ampenberger F, Gellert T et al. (2003). The Toll-like receptor 7 (TLR7)-specific stimulus loxoribine uncovers a strong relationship within the TLR7, 8 and 9 subfamily. Eur J Immunol 33: 2987–2997.
Heil F, Hemmi H, Hochrein H, Ampenberger F, Kirschning C, Akira S et al. (2004). Species-specific recognition of single-stranded RNA via Toll-like receptor 7 and 8. Science 303: 1526–1529.
Hemmi H, Kaisho T, Takeuchi O, Sato S, Sanjo H, Hoshino K et al. (2002). Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nat Immunol 3: 196–200.
Hengge UR, Ruzicka T . (2004). Topical immunomodulation in dermatology: potential of toll-like receptor agonists. Dermatol Surg 30: 1101–1112.
Hornung V, Guenthner-Biller M, Bourquin C, Ablasser A, Schlee M, Uematsu S et al. (2005). Sequence-specific potent induction of IFN-alpha by short interfering RNA in plasmacytoid dendritic cells through TLR7. Nat Med 11: 263–270.
Hurwitz DJ, Pincus L, Kupper TS . (2003). Imiquimod: a topically applied link between innate and acquired immunity. Arch Dermatol 139: 1347–1350.
Imbertson LM, Beaurline JM, Couture AM, Gibson SJ, Smith RMA, Miller RL et al. (1998). Cytokine induction in hairless mouse and rat skin after topical application of the immune response modifiers imiquimod and S-28463. J Invest Dermatol 110: 734–739.
Judge AD, Sood V, Shaw JR, Fang D, McClintock K, MacLachlan I . (2005). Sequence-dependent stimulation of the mammalian innate immune response by synthetic siRNA. Nat Biotechnol 23: 457–462.
Jurk M, Heil F, Vollmer J, Schetter C, Krieg AM, Wagner H et al. (2002). Human TLR7 or TLR8 independently confer responsiveness to the antiviral compound R-848. Nat Immunol 3: 499.
Jurk M, Kritzler A, Schulte B, Tluk S, Schetter C, Krieg AM et al. (2006). Modulating responsiveness of human TLR7 and 8 to small molecule ligands with T-rich phosphorothiate oligodeoxynucleotides. Eur J Immunol 36: 1815–1826.
Kamin A, Eigentler TK, Radny P, Bauer J, Weide B, Garbe C . (2005). Imiquimod in the treatment of extensive recurrent lentigo maligna. J Am Acad Dermatol 52 (Suppl. 1): 51–52.
Kariko K, Buckstein M, Ni H, Weissman D . (2005). Suppression of RNA recognition by Toll-like receptors: the impact of nucleoside modification and the evolutionary origin of RNA. Immunity 23: 165–175.
Karin M . (2006). Nuclear factor-kappaB in cancer development and progression. Nature 441: 431–436.
Korman N, Moy R, Ling M, Matheson R, Smith S, McKane S et al. (2005). Dosing with 5% imiquimod cream 3 times per week for the treatment of actinic keratosis: results of two phase 3, randomized, double-blind, parallel-group, vehicle-controlled trials. Arch Dermatol 141: 467–473.
Lebwohl M, Dinehart S, Whiting D, Lee PK, Tawfik N, Jorizzo J et al. (2004). Imiquimod 5% cream for the treatment of actinic keratosis: results from two phase III, randomized, double-blind, parallel group, vehicle-controlled trials. J Am Acad Dermatol 50: 714–721.
Lee J, Chuang TH, Redecke V, She L, Pitha PM, Carson DA et al. (2003). Molecular basis for immunostimulatory activity of guanine nucleoside analogs: activation of Toll-like receptor 7. Proc Natl Acad Sci USA 100: 6646–6651.
Levy O, Suter EE, Miller RL, Wessels MR . (2006). Unique efficacy of Toll-like receptor 8 agonists in activating human neonatal antigen-presenting cells. Blood 108: 1284–1289.
Lonsdale-Eccles AA, Morgan JM, Nagarajan S, Cruickshank DJ . (2006). Successful treatment of vulval melanoma in situ with topical 5% imiquimod cream. Br J Dermatol 155: 215–217.
Lore K, Betts MR, Brenchley JM, Kuruppu J, Khojasteh S, Perfetto S et al. (2003). Toll-like receptor ligands modulate dendritic cells to augment cytomegalovirus- and HIV-1-specific T cell responses. J Immunol 171: 4320–4328.
Lund JM, Alexopoulou L, Sato A, Karow M, Adams NC, Gale NW et al. (2004). Recognition of single-stranded RNA viruses by Toll-like receptor 7. Proc Natl Acad Sci USA 101: 5598–5603.
Ma Y, Pawar S, Sanchez-Schmitz G, Poisson L, Byers A, Shanen BC et al. (2007). In vitro vaccination site: a novel test bed for immunopotentiators. J Immunol 178: 36.30.
Megyeri K, Au W-C, Rosztoczy I . (1995). Stimulation of interferon and cytokine gene expression by imiquimod and stimulation by sendai virus utilize similar signal transduction pathways. Mol Cell Biol 15: 2207–2218.
Meyer T, Nindl I, Schmook T, Ulrich C, Sterry W, Stockfleth E . (2003). Induction of apoptosis by toll-like receptor-7 agonist in tissue cultures. Br J Dermatol 149: 9–14.
Miggin SM, O'Neill LAJ . (2006). New insights into the regulation of TLR signaling. J Leukoc Biol 80: 220–226.
Miller RL, Tomai MA, Harrison CJ, Bernstein DI . (2002). Immunomodulation as a treatment strategy for genital herpes: review of the evidence. Int Immunopharmacol 2: 443–451.
Navi D, Huntley A . (2004). Imiquimod 5% cream and the treatment of cutaneous malignancy. Dermatol Online J 10.
Nishiya T, DeFranco AL . (2004). Ligand-regulated chimeric receptor approach reveals distinctive subcellular localization and signaling properties of the Toll-like receptors. J Biol Chem 279: 19008–19017.
Nishiya T, Kajita E, Miwa S, DeFranco AL . (2005). TLR3 and TLR7 are targeted to the same intracellular compartments by distinct regulatory elements. J Biol Chem 280: 37107–37117.
Odashima M, Bamias G, Rivera-Nieves J, Linden J, Nast CC, Moskaluk CA et al. (2005). Activation of A2A adenosine receptor attenuates intestinal inflammation in animal models of inflammatory bowel disease. Gastroenterology 129: 26–33.
Palamara F, Meindl S, Holcmann M, Luhrs P, Stingl G, Sibilia M . (2004). Identification and characterization of pDC-like cells in normal mouse skin and melanomas treated with imiquimod. J Immunol 173: 3051–3061.
Patel K, Goodwin R, Chawla M, Laidler P, Price PE, Finlay AY et al. (2006). Imiquimod 5% cream monotherapy for cutaneous squamous cell carcinoma in situ (Bowen's disease): a randomized, double-blind, placebo-controlled trial. J Am Acad Dermatol 54: 1025–1032.
Peris K, Micantonio T, Fargnoli MC . (2003). Successful treatment of keratoacanthoma and actinic keratoses with imiquimod 5% cream. Eur J Dermatol 13: 413–414.
Peris K, Micantonio T, Fargnoli MC, Lozzi GP, Chimenti S . (2006). Imiquimod 5% cream in the treatment of Bowen's disease and invasive squamous cell carcinoma. J Am Acad Dermatol 55: 324–327.
Pope BL, MacIntyre JP, Kimball E, Lee S, Zhou L, Taylor GR et al. (1995). The immunostimulatory compound 7-allyl-8-oxoguanosine (loxoribine) induces a distinct subset of murine cytokines. Cell Immunol 162: 333–339.
Prins RM, Craft N, Bruhn KW, Khan-Farooqi H, Koya RC, Stripecke R et al. (2006). The TLR-7 agonist, imiquimod, enhances dendritic cell survival and promotes tumor antigen-specific T cell priming: relation to central nervous system antitumor immunity. J Immunol 176: 157–164.
Prinz BM, Hafner J, Dummer R, Burg G, Bruswanger U, Kempf W . (2004). Treatment of Bowen's disease with imiquimod 5% cream in transplant recipients. Transplantation 77: 790–791.
Qin J, Yao J, Cui G, Xiao H, Kim TW, Fraczek J et al. (2006). TLR8-mediated NF-kappaB and JNK activation are TAK1-independent and MEKK3-dependent. J Biol Chem 281: 21013–21021.
Ray CM, Kluk M, Grin CM, Grant-Kels JM . (2005). Successful treatment of malignant melanoma in situ with topical 5% imiquimod cream. Int J Dermatol 44: 428–434.
Rechtsteiner G, Warger T, Osterloh P, Schild H, Radsak MP . (2005). Cutting edge: priming of CTL by transcutaneous peptide immunization with imiquimod. J Immunol 174: 2476–2480.
Reiter MJ, Testerman TL, Miller RL, Weeks CE, Tomai MA . (1994). Cytokine induction in mice by the immunomodulator imiquimod. J Leukoc Biol 55: 234–240.
Roach JC, Glusman G, Rowen L, Kaur A, Purcell MK, Smith KD et al. (2005). The evolution of vertebrate Toll-like receptors. Proc Natl Acad Sci USA 102: 9577–9578.
Roseeuw D . (2003). The treatment of basal skin carcinomas in two sisters with Xeroderma pigmentosum. Clin Exp Dermatol 28 (Suppl. 1): 30–32.
Sauder DN, Smith MH, Senta-McMillian T, Soria I, Meng TC . (2003). Randomized, single-blind, placebo-controlled study of topical application of the immune response modulator resiquimod in healthy adults. Antimicrob Agents Chemother 47: 3846–3852.
Schön M, Bong AB, Drewniok C, Herz J, Geilen CC, Reifenberger J et al. (2003). Tumor-selective induction of apoptosis and the small-molecule immune response modifier imiquimod. J Natl Cancer Inst 95: 1138–1149.
Schön M, Schön MP . (2007). The antitumoral mode of action of imiquimod and other imidazoquinolines. Curr Med Chem 14: 681–687.
Schön MP, Schön M, Klotz KN . (2006). The small antitumoral immune response modifier imiquimod interacts with adenosine receptor signaling in a TLR7- and TLR8-independent fashion. J Invest Dermatol 126: 1338–1347.
Schön MP, Wienrich BG, Drewniok C, Bong AB, Eberle J, Geilen CC et al. (2004). Death receptor-independent apoptosis in malignant melanoma induced by the small-molecule immune response modifier imiquimod. J Invest Dermatol 122: 1266–1276.
Schulze HJ, Cribier B, Requena L, Reifenberger J, Ferrandiz C, Garcia Diez A et al. (2005). Imiquimod 5% cream for the treatment of superficial basal cell carcinoma: results from a randomized vehicle-controlled phase III study in Europe. Br J Dermatol 152: 939–947.
Schwarz K, Storni T, Manolova V, Didierlaurent A, Sirard JC, Rothlisberger P et al. (2003). Role of toll-like receptors in costimulating cytotoxic T cell responses. Eur J Immunol 33: 1465–1470.
Shackleton M, Davis ID, Hopkins W, Jackson H, Dimopoulos N, Tai T et al. (2004). The impact of imiquimod, a toll-like receptor-7 ligand (TLR7L), on the immunogenicity of melanoma peptide vaccination with adjuvant Flt3 ligand. Cancer Immun 23: 9.
Sidbury R, Neuschler N, Neuschler E, Sun P, Wang XQ, Miller RL et al. (2003). Topically applied imiquimod inhibits vascular tumor growth in vivo. J Invest Dermatol 121: 1205–1209.
Sidky YA, Borden EC, Weeks CE, Reiter MJ, Hatcher JF, Bryan GT . (1992). Inhibition of murine tumor growth by an interferon-inducing imidazoquinoline. Cancer Res 52: 3528–3533.
Smith KJ, Germain M, Skelton H . (2001). Squamous cell carcinoma in situ (Bowen's disease) in renal transplant patients treated with 5% imiquimod and 5% 5-fluorouracil therapy. Dermatol Surg 27: 561–564.
Stanley MA . (2002). Imiquimod and the imidazoquinolones: mechanism of action and therapeutic potential. Clin Exp Dermatol 27: 571–577.
Stary G, Bangert C, Tauber M, Strohal R, Kopp T, Stingl G . (2007). Tumoricidal activity of TLR7/8-activated inflammatory dendritic cells. J Exp Med 204: 1441–1451.
Steinmann A, Funk JO, Schuler G, von den Driesch P . (2000). Topical imiquimod treatment of a cutaneous melanoma metastasis. J Am Acad Dermatol 43: 555–556.
Stephanou A, Latchman DS . (2005). Opposing actions of STAT-1 and STAT-3. Growth Factors 23: 177–182.
Sterry W, Ruzicka T, Herrera E, Takwale A, Bichel J, Andres K et al. (2002). Imiquimod 5% cream for the treatment of superficial and nodular basal cell carcinoma: randomized studies comparing low-frequency dosing with and without occlusion. Br J Dermatol 147: 1227–1236.
Stockfleth E, Meyer T, Benninghoff B, Christophers E . (2001). Successful treatment of actinic keratosis with imiquimod cream 5%: a report of six cases. Br J Dermatol 144: 1050–1053.
Stockfleth E, Meyer T, Benninghoff B, Salasche S, Papadopoulos L, Ulrich C et al. (2002). A randomized, double-blind, vehicle-controlled study to assess 5% imiquimod cream for the treatment of multiple actinic keratoses. Arch Dermatol 138: 1498–1502.
Suchin KR, Junkins-Hopkins JM, Rook AH . (2002). Treatment of stage IA cutaneous T-cell lymphoma with topical application of the immune response modifier imiquimod. Arch Dermatol 138: 1137–1139.
Sullivan TP, Dearaujo T, Vincek V, Berman B . (2003). Evaluation of superficial basal cell carcinomas after treatment with imiquimod 5% cream or vehicle for apoptosis and lymphocyte phenotyping. Dermatol Surg 29: 1181–1186.
Suzuki H, Wang B, Shivji GM, Toto P, Amerio P, Tomai MA et al. (2000). Imiquimod, a topical immune response modifier, induces migration of Langerhans cells. J Invest Dermatol 114: 135–141.
Szeimies RM, Gerritsen MJ, Gupta G, Ortonne JP, Serresi S, Bichel J et al. (2004). Imiquimod 5% cream for the treatment of actinic keratosis: results from a phase III, randomized, double-blind, vehicle-controlled, clinical trial with histology. J Am Acad Dermatol 51: 547–555.
Thomsen LL, Topley P, Daly MG, Brett SJ, Tite JP . (2004). Imiquimod and resiquimod in a mouse model: adjuvants for DNA vaccination by particle-mediated immunotherapeutic delivery. Vaccine 22: 1799–1809.
Tomai MA, Imbertson LM, Stanczak TL, Tygrett LT, Waldschmidt TJ . (2000). The immune response modifiers imiquimod and R-848 are potent activators of B lymphocytes. Cell Immunol 203: 55–65.
Ugurel S, Wagner A, Pföhler C, Tilgen W, Reinhold U . (2002). Topical imiquimod eradicates skin metastases of malignant melanoma but fails to prevent rapid lymphogenous metastatic spread. Br J Dermatol 147: 621–624.
Urosevic M, Dummer R, Conrad C, Beyeler M, Laine E, Burg G et al. (2005). Disease-independent skin recruitment and activation of plasmacytoid predendritic cells following imiquimod treatment. J Natl Cancer Inst 97: 1143–1153.
Urosevic M, Maier T, Benninghoff B, Slade HB, Burg G, Dummer R . (2003). Mechanisms underlying imiquimod-induced regression of basal cell carcinomas in vivo. Arch Dermatol 139: 1325–1332.
Urosevic M, Oberholzer PA, Maier T, Hafner J, Laine E, Slade HB et al. (2004). Imiquimod treatment induces expression of opioid growth factor receptor: a novel tumor antigen induced by interferon-α? Clin Cancer Res 10: 4959–4970.
Vidal D, Matias-Guiu X, Alomar A . (2004). Efficacy of imiquimod for the expression of Bcl-2, Ki67, p53 and basal cell carcinoma apoptosis. Br J Dermatol 151: 656–662.
Wagner TL, Ahonen CL, Couture AM, Gibson SJ, Miller RL, Smith RM et al. (1999). Modulation of TH1 and TH2 cytokine production with the immune response modifiers, R-848 and imiquimod. Cell Immunol 191: 10–19.
Wagner TL, Horton VL, Carlson GL . (1997). Induction of cytokines in Cynomolgus monkeys by the immune response modifiers, imiquimod, S-27609 and S-28463. Cytokine 9: 837–845.
Weeks CE, Gibson SJ . (1994). Induction of interferon and other cytokines by imiquimod and its hydroxylated metabolite R-842 in human blood cells in vitro. J Interferon Cytokine Res 14: 81–85.
Wille-Reece U, Flynn BJ, Lore K, Koup RA, Miles AP, Saul A et al. (2006). Toll-like receptor agonists influence the magnitude and quality of memory T cell responses after prime-boost immunization in nonhuman primates. J Exp Med 203: 1249–1258.
Wolf IH, Cerroni L, Kodama K, Kerl H . (2005). Treatment of lentigo maligna (melanoma in situ) with the immune response modifier imiquimod. Arch Dermatol 141: 510–514.
Wolf IH, Smolle J, Binder B, Cerroni L, Richtig E, Kerl H . (2003). Topical imiquimod in the treatment of metastatic melanoma to skin. Arch Dermatol 139: 273–276.
Zeitouni NC, Dawson K, Cheney RT . (2005). Treatment of cutaneous metastatic melanoma with imiquimod 5% cream and the pulsed-dye laser. Br J Dermatol 152: 376–377.
Zuber AK, Brave A, Engstrom G, Zuber B, Ljungberg K, Fredriksson M et al. (2004). Topical delivery of imiquimod to a mouse model as a novel adjuvant for human immunodeficiency virus (HIV) DNA. Vaccine 22: 1791–1798.
Acknowledgements
This work was supported by a research grant from the Dr Mildred Scheel Stiftung/Deutsche Krebshilfe (10-2196 Schö-2) to MS and MPS, and by a Rudolf Virchow Award from the Deutsche Forschungsgemeinschaft to MPS.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schön, M., Schön, M. TLR7 and TLR8 as targets in cancer therapy. Oncogene 27, 190–199 (2008). https://doi.org/10.1038/sj.onc.1210913
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1210913
Keywords
This article is cited by
-
E3 ligase RNF99 negatively regulates TLR-mediated inflammatory immune response via K48-linked ubiquitination of TAB2
Cell Death & Differentiation (2023)
-
Translocation of intracellular CD24 constitutes a triggering event for drug resistance in breast cancer
Scientific Reports (2021)
-
Development of a therapeutic vaccine targeting Merkel cell polyomavirus capsid protein VP1 against Merkel cell carcinoma
npj Vaccines (2021)
-
Promoter G-quadruplexes and transcription factors cooperate to shape the cell type-specific transcriptome
Nature Communications (2021)
-
Intratumoral immunotherapy using platelet-cloaked nanoparticles enhances antitumor immunity in solid tumors
Nature Communications (2021)