-
PDF
- Split View
-
Views
-
Cite
Cite
M. C. Kapetanovic, T. Saxne, A. Sjöholm, L. Truedsson, G. Jönsson, P. Geborek, Influence of methotrexate, TNF blockers and prednisolone on antibody responses to pneumococcal polysaccharide vaccine in patients with rheumatoid arthritis, Rheumatology, Volume 45, Issue 1, January 2006, Pages 106–111, https://doi.org/10.1093/rheumatology/kei193
Close - Share Icon Share
Abstract
Objective. To compare antibody responses to 23-valent pneumococcal vaccine (Pneumovax®) in controls and patients with established rheumatoid arthritis (RA) treated with TNF blockers, methotrexate (MTX) or a combination of both.
Methods. Patients with RA (n = 149) and healthy controls (n = 47) were vaccinated. Treatment with TNF blockers (etanercept or infliximab) and MTX was given to 50 patients, and 62 patients were treated with TNF blockers alone or with other DMARDs. MTX alone was given to 37 patients. Concentrations of immunoglobulin G (IgG) antibodies against pneumococcal capsular polysaccharides 23F and 6B were measured by enzyme-linked immunoassay before and 4–6 weeks after vaccination. An immune response was defined as a twofold or higher increase in antibody concentration following vaccination.
Results. Prevaccination antibody levels for both 23F and 6B were similar in the patient groups. Antibody concentrations after vaccination increased significantly in all groups. Patients treated with TNF blockers without MTX showed better immune responses than those treated with TNF blockers in combination with MTX (P = 0.037 for 23F and P = 0.004 for 6B) or MTX alone (P<0.001 for both 23F and 6B). RA patients given MTX alone had the lowest immune responses. Prednisolone treatment did not influence the responses.
Conclusions. Patients treated with TNF blockers and controls showed similar responses to vaccination. In contrast, patients treated with MTX had reduced responses regardless of anti-TNF treatment. The findings do not argue against the use of pneumococcal vaccination in RA patients undergoing treatment with TNF blockers.
Infection with Streptococcus pneumoniae is a major cause of mortality and morbidity throughout the world. To prevent severe pneumococcal infections and their complications, the Swedish National Board of Health and Welfare and the CDC Advisory Committee on Immunization Practice recommend immunization with 23-valent pneumococcal polysaccharide vaccine in persons >65 years of age and patients suffering from chronic illness at high risk of invasive pneumococcal disease [1–3].
In patients with rheumatoid arthritis (RA), infections cause significant morbidity and mortality. Possible explanations include immune dysfunction associated with the disease itself, co-morbid illnesses and/or concomitant medication such as immunosuppressive drugs, including long-term systemic glucocorticoids [4–10]. The incidence of objectively confirmed infections in patients with RA was found to be increased compared with age- and sex-matched subjects without RA. Also, infections requiring hospitalization, including bacteraemia/septicaemia and pneumonia, were reported to be significantly more frequent in RA patients [4]. The use of disease-modifying anti-rheumatic drugs (DMARDs), including concomitant treatment with glucocorticoids, has been found to be associated with increased risk of infections [7].
Recently, the introduction of anti-TNF treatments has contributed to a somewhat changed pattern of infections in RA [11–13]. More common infections, such as upper respiratory tract infections, have also been described among the common adverse events and reasons for withdrawal of anti-TNF therapy in clinical trials as well as in observational studies [8, 14]. Doubled rates of serious infectious have also been reported [15]. Case reports of serious infections due to S. pneumoniae during anti-TNF therapy include pneumonia, severe pneumonia and necrotizing fasciitis (infliximab) [16, 17], and fatal septicaemia (etanercept) [18].
Commercially available 23-valent pneumococcal capsular polysaccharide vaccine contains 23 purified capsular polysaccharide antigens of S. pneumoniae and the vaccine covers at least 85–90% of the serotypes causing invasive pneumococcal infections [2]. The eight most common serotypes of invasive S. pneumoniae isolates reported to the Swedish Institute for Infectious Disease Control during 1988–1998 were serotypes 14, 7F, 9V, 4, 3, 1, 23F and 6B [19].
Applying the CDC Advisory Committee on Immunization Practice recommendations to patients with rheumatic disease, pneumococcal vaccination should be considered and encouraged in a majority of the patients in spite of the lack of convincing clinical evidence of efficacy [1, 2, 20–29]. Also, in immunocompromised patients there is evidence of diminished or absent antibody responses to pneumococcal vaccine in certain groups [2, 30–35].
There are insufficient data from larger controlled trials on RA patients undergoing treatment with TNF-blocking agents and other DMARDs to allow more specific recommendations concerning pneumococcal vaccination. Despite the presumably decreased effectiveness of pneumococcal vaccine in some immunocompromised patients, the potential benefits have so far been judged to justify its use. This study aims to assess the effects of different therapy modalities, including TNF blockers, on the anti-capsular antibody response to vaccination in patients with longstanding RA and to compare the response obtained in healthy controls.
Material and methods
All patients treated with TNF blockers at the Department of Rheumatology at Lund University Hospital were offered pneumococcal vaccination without extra cost. To identify control groups, consecutive RA patients taking methotrexate (MTX) without anti-TNF drugs attending the Department of Rheumatology, as well as healthy individuals among staff members (controls) at the Departments of Rheumatology and Infectious Diseases at Lund University Hospital, were offered free vaccination. The study was conducted during the winter seasons 2000/2001 and 2001/2002. Patients were offered pneumococcal vaccination according to the guidelines of the Swedish National Health Board. Ethical approval from local Ethical Review Board at Lund University (LU 513-01) was obtained for vaccination of the medical staff.
Etanercept was given in a dosage of 25 mg subcutaneously twice a week and infliximab as an intravenous infusion in a dosage of 3 mg/kg body weight over 2 h at the start, after 2 and 6 weeks and thereafter as a rule every 8 weeks. The treatments were given on an out-patient basis. For comparison, anti-TNF-treated patients were stratified into two groups according to concomitant use of MTX. A group of 62 patients were treated with TNF blockers as monotherapy or combined with DMARDs other than MTX (‘TNF blockers without MTX’ group). Fourteen of these patients received one other DMARD: four sulphasalazine, five azathioprine, two antimalarial, two oral gold, and one cyclosporin. Fifty patients were treated with anti-TNF treatment combined with MTX (‘TNF blockers + MTX’ group) and 12 of these patients had concomitant therapy with other DMARDs: six sulphasalazine, two cyclosporin, two antimalarial and two both sulphasalazine and antimalarial. In the group of patients treated with MTX without TNF blockers, 10 patients received concomitant DMARDs (five sulphasalazine and five antimalarial). The protocol also included items of diagnosis, age, disease duration, use and dosage of prednisolone and other DMARDs for all patients.
Vaccination procedure
Each participant received a commercially available 23-valent polysaccharide pneumococcal vaccine (Pneumovax; Merck) administrated as a subcutaneous injection in the upper arm with 0.5 ml of a single lot of pneumococcal vaccine containing 25 μg of each of 23 capsular polysaccharide types. Blood samples were obtained before and 4–6 weeks after vaccination and serum was frozen at −20°C. Vaccination in infliximab-treated patients was performed immediately prior to a scheduled infliximab infusion.
Quantitation of human IgG antibodies specific for S. pneumoniae capsular polysaccharides by ELISA
The results are presented as the immunization response, i.e. the ratio between post- and prevaccination antibody concentrations. A positive immunization response was defined as a 2-fold or higher increase compared with the prevaccination antibody concentration. Since a protective level of serum antibody has not been strictly defined and may differ among serotypes, these values were not used to identify individuals with probable/possible protective antibody levels.
Levels of serotype-specific pneumococcal IgG to 23F and 6B were measured using the WHO standard enzyme-linked immunosorbent assay (ELISA) for quantitation of human IgG antibodies specific for S. pneumoniae capsular polysaccharides (Pn PS ELISA), as previously described [39]. Briefly, ELISA plates were coated with Pn PS. Dilutions of human sera absorbed with pneumococcal capsular polysaccharide were then added to the ELISA plates. The serotype-specific antibodies (for 23F and 6B) were detected using goat anti-human IgG antibodies conjugated with alkaline phosphatase, followed by addition of the substrate, p-nitrophenyl phosphate. The optical density was measured at 405 nm using an ELISA plate reader. The optical density of the coloured end product is proportional to the amount of anti-capsular PS present in the serum. The assay was calibrated with an international reference serum that was kindly provided by Dr C. Frasch, Bethesda, MD, USA [40]. The lower limit of detection was 0.01 mg/l.
The distributions of the outcome variables were extremely skewed (Shapiro–Wilk W = 0.24–0.74). Therefore, we used non-parametric methods: the Mann–Whitney U-test for comparisons between groups, Wilcoxon's test for paired variables, and the χ2 test for ordinal variables. P values <0.05 were considered significant.
Results
Altogether, 149 patients with established RA and 47 healthy volunteers participated. Patients with ongoing anti-TNF treatment (n = 112) with either etanercept (n = 48) or infliximab (n = 64) and patients with MTX therapy (n = 37) without anti-TNF drugs were vaccinated. Demographic and clinical characteristics of patients and healthy volunteers prior to vaccination are summarized in Table 1. Etanercept was more commonly used without MTX compared with infliximab. Controls were younger and patients in the MTX group were older. Disease duration was longer for TNF blockers without MTX. There were no differences in MTX dosage and duration of MTX treatment before vaccination, and anti-TNF treatment duration was similar between the groups receiving TNF blockers without MTX and TNF blockers + MTX. The number of patients taking prednisolone was similar in all patient groups, and the dosages were not significantly different between the groups. Gender was not significantly different between any of the groups. Disease activity at vaccination was similar in all patient groups, but, compared with the time when anti-TNF was initiated, there had been a significant reduction in the number of patients with high disease activity.
Demographics and clinical characteristics of patients and healthy controls before vaccination
| . | Treatment . | . | . | . | . | |||
|---|---|---|---|---|---|---|---|---|
| . | I: TNF blockers without MTX . | II: TNF blockers + MTX . | III: MTX . | IV: controls . | Significant differences between groups . | |||
| Number | 62 | 50 | 37 | 47 | ||||
| Infliximab/etanercept (number) | 27/35 | 37/13 | P = 0.001 for I↔II | |||||
| Age (yr)a | 53.7 (15.1–85.3) | 52.8 (20.9–80.8) | 61.3 (20.8–81.4) | 30.3 (19.2–60.3) | P<0.001 for I↔IV, II↔IV, and III↔IV | |||
| Disease duration (yr)a | 20.8 (1.5–55.9) | 10.8 (2.4–39.8) | 7.0 (0.9–46.9) | P<0.001 for I↔III and II↔III | ||||
| Female (%) | 76 | 70 | 68 | 74 | P<0.001 for I↔II and I↔III | |||
| Concomitant steroids (%) | 50.0 | 52.0 | 51.4 | |||||
| Prednisolone dosage (mg/week)a | 17.5 (0–105) | 6.5 (0–105) | 17.5 (0–105) | |||||
| Anti-TNF treatment duration (yr) median (min.−max.)a | 1.1 (0–1.6) | 0.7 (0–1.7) | ||||||
| Methotrexate treatment duration (yr)a | 3.5 (0–10.9) | 2.9 (0.1–13.0) | ||||||
| Methotrexate dosage (mg/week)a | 15 (5–25) | 15 (7.5–25) | ||||||
| DASb | ||||||||
| Low/intermediate/high disease activity at vaccination (%) | 49/41/10 | 50/44/6 | 53/35/12 | |||||
| Low/intermediate/high disease activity at anti-TNF initiation (%) | 6/54/40 | 6/54/40 | ||||||
| . | Treatment . | . | . | . | . | |||
|---|---|---|---|---|---|---|---|---|
| . | I: TNF blockers without MTX . | II: TNF blockers + MTX . | III: MTX . | IV: controls . | Significant differences between groups . | |||
| Number | 62 | 50 | 37 | 47 | ||||
| Infliximab/etanercept (number) | 27/35 | 37/13 | P = 0.001 for I↔II | |||||
| Age (yr)a | 53.7 (15.1–85.3) | 52.8 (20.9–80.8) | 61.3 (20.8–81.4) | 30.3 (19.2–60.3) | P<0.001 for I↔IV, II↔IV, and III↔IV | |||
| Disease duration (yr)a | 20.8 (1.5–55.9) | 10.8 (2.4–39.8) | 7.0 (0.9–46.9) | P<0.001 for I↔III and II↔III | ||||
| Female (%) | 76 | 70 | 68 | 74 | P<0.001 for I↔II and I↔III | |||
| Concomitant steroids (%) | 50.0 | 52.0 | 51.4 | |||||
| Prednisolone dosage (mg/week)a | 17.5 (0–105) | 6.5 (0–105) | 17.5 (0–105) | |||||
| Anti-TNF treatment duration (yr) median (min.−max.)a | 1.1 (0–1.6) | 0.7 (0–1.7) | ||||||
| Methotrexate treatment duration (yr)a | 3.5 (0–10.9) | 2.9 (0.1–13.0) | ||||||
| Methotrexate dosage (mg/week)a | 15 (5–25) | 15 (7.5–25) | ||||||
| DASb | ||||||||
| Low/intermediate/high disease activity at vaccination (%) | 49/41/10 | 50/44/6 | 53/35/12 | |||||
| Low/intermediate/high disease activity at anti-TNF initiation (%) | 6/54/40 | 6/54/40 | ||||||
Groups were as follows: TNF blockers without MTX = anti-TNF treatment with or without DMARD excluding MTX; TNF blockers + MTX = anti-TNF treatment plus MTX with or without other DMARD; MTX = MTX with or without other DMARD excluding TNF blockers; controls = healthy controls. aMedian (minimum–maximum). bDAS, disease activity score: low, DAS<3.2; intermediate, DAS 3.2–5.1; high DAS>5.1 using the three variables 28-joint swollen joint count (SJC), 28-joint tender joint count (TJC) and CRP in mg/l, where DAS = [√(TJC) × 0.56 + √(SJC) × 0.28 + 0.36 ln(CRP + 1)] × 1.1 + 1.15.
Demographics and clinical characteristics of patients and healthy controls before vaccination
| . | Treatment . | . | . | . | . | |||
|---|---|---|---|---|---|---|---|---|
| . | I: TNF blockers without MTX . | II: TNF blockers + MTX . | III: MTX . | IV: controls . | Significant differences between groups . | |||
| Number | 62 | 50 | 37 | 47 | ||||
| Infliximab/etanercept (number) | 27/35 | 37/13 | P = 0.001 for I↔II | |||||
| Age (yr)a | 53.7 (15.1–85.3) | 52.8 (20.9–80.8) | 61.3 (20.8–81.4) | 30.3 (19.2–60.3) | P<0.001 for I↔IV, II↔IV, and III↔IV | |||
| Disease duration (yr)a | 20.8 (1.5–55.9) | 10.8 (2.4–39.8) | 7.0 (0.9–46.9) | P<0.001 for I↔III and II↔III | ||||
| Female (%) | 76 | 70 | 68 | 74 | P<0.001 for I↔II and I↔III | |||
| Concomitant steroids (%) | 50.0 | 52.0 | 51.4 | |||||
| Prednisolone dosage (mg/week)a | 17.5 (0–105) | 6.5 (0–105) | 17.5 (0–105) | |||||
| Anti-TNF treatment duration (yr) median (min.−max.)a | 1.1 (0–1.6) | 0.7 (0–1.7) | ||||||
| Methotrexate treatment duration (yr)a | 3.5 (0–10.9) | 2.9 (0.1–13.0) | ||||||
| Methotrexate dosage (mg/week)a | 15 (5–25) | 15 (7.5–25) | ||||||
| DASb | ||||||||
| Low/intermediate/high disease activity at vaccination (%) | 49/41/10 | 50/44/6 | 53/35/12 | |||||
| Low/intermediate/high disease activity at anti-TNF initiation (%) | 6/54/40 | 6/54/40 | ||||||
| . | Treatment . | . | . | . | . | |||
|---|---|---|---|---|---|---|---|---|
| . | I: TNF blockers without MTX . | II: TNF blockers + MTX . | III: MTX . | IV: controls . | Significant differences between groups . | |||
| Number | 62 | 50 | 37 | 47 | ||||
| Infliximab/etanercept (number) | 27/35 | 37/13 | P = 0.001 for I↔II | |||||
| Age (yr)a | 53.7 (15.1–85.3) | 52.8 (20.9–80.8) | 61.3 (20.8–81.4) | 30.3 (19.2–60.3) | P<0.001 for I↔IV, II↔IV, and III↔IV | |||
| Disease duration (yr)a | 20.8 (1.5–55.9) | 10.8 (2.4–39.8) | 7.0 (0.9–46.9) | P<0.001 for I↔III and II↔III | ||||
| Female (%) | 76 | 70 | 68 | 74 | P<0.001 for I↔II and I↔III | |||
| Concomitant steroids (%) | 50.0 | 52.0 | 51.4 | |||||
| Prednisolone dosage (mg/week)a | 17.5 (0–105) | 6.5 (0–105) | 17.5 (0–105) | |||||
| Anti-TNF treatment duration (yr) median (min.−max.)a | 1.1 (0–1.6) | 0.7 (0–1.7) | ||||||
| Methotrexate treatment duration (yr)a | 3.5 (0–10.9) | 2.9 (0.1–13.0) | ||||||
| Methotrexate dosage (mg/week)a | 15 (5–25) | 15 (7.5–25) | ||||||
| DASb | ||||||||
| Low/intermediate/high disease activity at vaccination (%) | 49/41/10 | 50/44/6 | 53/35/12 | |||||
| Low/intermediate/high disease activity at anti-TNF initiation (%) | 6/54/40 | 6/54/40 | ||||||
Groups were as follows: TNF blockers without MTX = anti-TNF treatment with or without DMARD excluding MTX; TNF blockers + MTX = anti-TNF treatment plus MTX with or without other DMARD; MTX = MTX with or without other DMARD excluding TNF blockers; controls = healthy controls. aMedian (minimum–maximum). bDAS, disease activity score: low, DAS<3.2; intermediate, DAS 3.2–5.1; high DAS>5.1 using the three variables 28-joint swollen joint count (SJC), 28-joint tender joint count (TJC) and CRP in mg/l, where DAS = [√(TJC) × 0.56 + √(SJC) × 0.28 + 0.36 ln(CRP + 1)] × 1.1 + 1.15.
Antibody response
Prior to vaccination almost all participants had detectable levels of antibodies to both 23F and 6B (only two lacked antibodies to 23F, and seven to 6B). Prevaccination antibody concentrations were similar for the different treatment groups but tended to be higher among controls for both 23F and 6B (Table 2). The only significant differences were for 23F between the TNF blockers without MTX group vs controls (P = 0.035) and the TNF blockers + MTX group vs controls (P = 0.050).
Prevaccination and postvaccination antibody concentrations (mg/l) for 23F and 6B
| . | I: TNF blockers without MTX . | II: TNF blockers + MTX . | III: MTX . | IV: Controls . | Significant differences between groups . | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Prevaccination antibody concentrations | ||||||||||
| 23F | 0.7 (0–9.4) | 0.8 (0–4.7) | 0.8 (0.1–3.8) | 1.2 (0–9.7) | I↔IV P = 0.035; | |||||
| II↔IV P = 0.050 | ||||||||||
| 6B | 0.9 (0–21) | 1.0 (0–7.8) | 1.1 (0–8.0) | 2.1 (0.1–30) | ||||||
| Postvaccination antibody concentrations | ||||||||||
| 23F | 2.4 (0.2–18) | 1.7 (0.1–19) | 1.6 (0–9.1) | 2.8 (0.0–103) | I↔III P= 0.036; | |||||
| II↔IV P = 0.030; | ||||||||||
| III↔IV P = 0.017 | ||||||||||
| 6B | 3.8 (0–97) | 3.9 (0.1–63) | 2.1 (0–33) | 4.6 (0.1–101) | III↔IV P = 0.040 | |||||
| Immunization responsea | ||||||||||
| 23F | 2.8 (0.9–68) | 2.0 (0.7–36) | 1.4 (0.3–15) | 2.3 (0.2–91) | I↔II P = 0.037; | |||||
| I↔III P<0.001 | ||||||||||
| 6B | 3.4 (0.8–280) | 1.8 (0.9–44) | 1.6 (0.8–20) | 2.2 (0.4–75) | I↔II P = 0.004; | |||||
| I↔III P<0.001; | ||||||||||
| I↔IV P = 0.047 | ||||||||||
| . | I: TNF blockers without MTX . | II: TNF blockers + MTX . | III: MTX . | IV: Controls . | Significant differences between groups . | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Prevaccination antibody concentrations | ||||||||||
| 23F | 0.7 (0–9.4) | 0.8 (0–4.7) | 0.8 (0.1–3.8) | 1.2 (0–9.7) | I↔IV P = 0.035; | |||||
| II↔IV P = 0.050 | ||||||||||
| 6B | 0.9 (0–21) | 1.0 (0–7.8) | 1.1 (0–8.0) | 2.1 (0.1–30) | ||||||
| Postvaccination antibody concentrations | ||||||||||
| 23F | 2.4 (0.2–18) | 1.7 (0.1–19) | 1.6 (0–9.1) | 2.8 (0.0–103) | I↔III P= 0.036; | |||||
| II↔IV P = 0.030; | ||||||||||
| III↔IV P = 0.017 | ||||||||||
| 6B | 3.8 (0–97) | 3.9 (0.1–63) | 2.1 (0–33) | 4.6 (0.1–101) | III↔IV P = 0.040 | |||||
| Immunization responsea | ||||||||||
| 23F | 2.8 (0.9–68) | 2.0 (0.7–36) | 1.4 (0.3–15) | 2.3 (0.2–91) | I↔II P = 0.037; | |||||
| I↔III P<0.001 | ||||||||||
| 6B | 3.4 (0.8–280) | 1.8 (0.9–44) | 1.6 (0.8–20) | 2.2 (0.4–75) | I↔II P = 0.004; | |||||
| I↔III P<0.001; | ||||||||||
| I↔IV P = 0.047 | ||||||||||
aRatio of post- and prevaccination concentrations. All values are given as median (minimum−maximum) values. All values <0.01 mg/l are taken as 0.
Prevaccination and postvaccination antibody concentrations (mg/l) for 23F and 6B
| . | I: TNF blockers without MTX . | II: TNF blockers + MTX . | III: MTX . | IV: Controls . | Significant differences between groups . | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Prevaccination antibody concentrations | ||||||||||
| 23F | 0.7 (0–9.4) | 0.8 (0–4.7) | 0.8 (0.1–3.8) | 1.2 (0–9.7) | I↔IV P = 0.035; | |||||
| II↔IV P = 0.050 | ||||||||||
| 6B | 0.9 (0–21) | 1.0 (0–7.8) | 1.1 (0–8.0) | 2.1 (0.1–30) | ||||||
| Postvaccination antibody concentrations | ||||||||||
| 23F | 2.4 (0.2–18) | 1.7 (0.1–19) | 1.6 (0–9.1) | 2.8 (0.0–103) | I↔III P= 0.036; | |||||
| II↔IV P = 0.030; | ||||||||||
| III↔IV P = 0.017 | ||||||||||
| 6B | 3.8 (0–97) | 3.9 (0.1–63) | 2.1 (0–33) | 4.6 (0.1–101) | III↔IV P = 0.040 | |||||
| Immunization responsea | ||||||||||
| 23F | 2.8 (0.9–68) | 2.0 (0.7–36) | 1.4 (0.3–15) | 2.3 (0.2–91) | I↔II P = 0.037; | |||||
| I↔III P<0.001 | ||||||||||
| 6B | 3.4 (0.8–280) | 1.8 (0.9–44) | 1.6 (0.8–20) | 2.2 (0.4–75) | I↔II P = 0.004; | |||||
| I↔III P<0.001; | ||||||||||
| I↔IV P = 0.047 | ||||||||||
| . | I: TNF blockers without MTX . | II: TNF blockers + MTX . | III: MTX . | IV: Controls . | Significant differences between groups . | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Prevaccination antibody concentrations | ||||||||||
| 23F | 0.7 (0–9.4) | 0.8 (0–4.7) | 0.8 (0.1–3.8) | 1.2 (0–9.7) | I↔IV P = 0.035; | |||||
| II↔IV P = 0.050 | ||||||||||
| 6B | 0.9 (0–21) | 1.0 (0–7.8) | 1.1 (0–8.0) | 2.1 (0.1–30) | ||||||
| Postvaccination antibody concentrations | ||||||||||
| 23F | 2.4 (0.2–18) | 1.7 (0.1–19) | 1.6 (0–9.1) | 2.8 (0.0–103) | I↔III P= 0.036; | |||||
| II↔IV P = 0.030; | ||||||||||
| III↔IV P = 0.017 | ||||||||||
| 6B | 3.8 (0–97) | 3.9 (0.1–63) | 2.1 (0–33) | 4.6 (0.1–101) | III↔IV P = 0.040 | |||||
| Immunization responsea | ||||||||||
| 23F | 2.8 (0.9–68) | 2.0 (0.7–36) | 1.4 (0.3–15) | 2.3 (0.2–91) | I↔II P = 0.037; | |||||
| I↔III P<0.001 | ||||||||||
| 6B | 3.4 (0.8–280) | 1.8 (0.9–44) | 1.6 (0.8–20) | 2.2 (0.4–75) | I↔II P = 0.004; | |||||
| I↔III P<0.001; | ||||||||||
| I↔IV P = 0.047 | ||||||||||
aRatio of post- and prevaccination concentrations. All values are given as median (minimum−maximum) values. All values <0.01 mg/l are taken as 0.
Postvaccination antibody concentrations increased significantly in all groups. Median and range values as well as significant differences between the treatment groups are given in Table 2.
Immunization responses, i.e. the ratios between post- and prevaccination antibody concentrations, are summarized in Table 2. There were large differences between the groups with regard to immune responses. Responses were highest for TNF blockers without MTX and lowest for MTX. Controls tended to respond better than patients in the MTX group, but this did not reach significance (P = 0.059 for 23F and P = 0.058 for 6B). There were no significant differences between the TNF blockers + MTX and MTX groups (P = 0.065 for 23F and P = 0.246 for 6B).
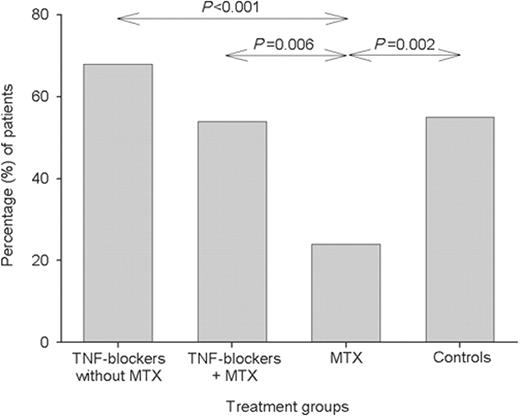
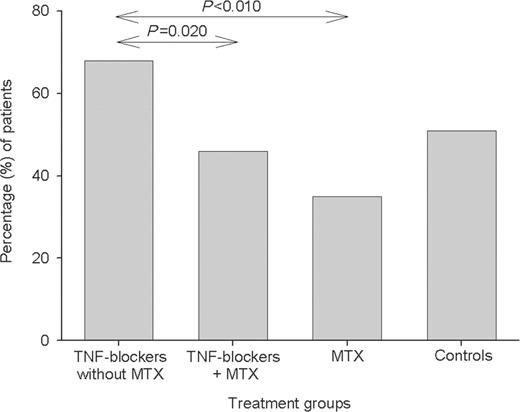
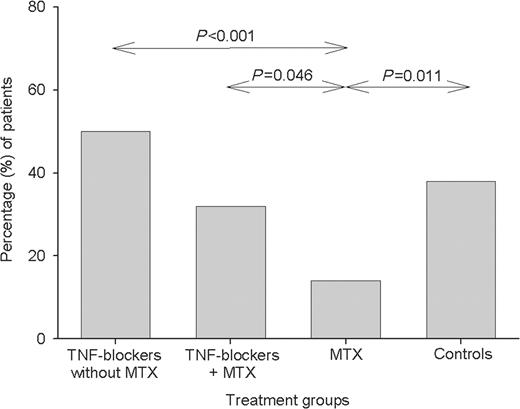
The proportion of subjects having an immune response, defined as a ≥2-fold increase in the antibody concentration, differed between the treatment groups (Figs 1–3), but essentially followed the same patterns as for immune response ratios. The highest percentage of immune responses was found in the group receiving TNF blockers without MTX and the lowest in the MTX group.
Percentage of patients with immune response, defined as a ≥2-fold increase in antibody level to 23F. Groups were as follows: TNF blockers without MTX = anti-TNF treatment with or without DMARDs excluding MTX; TNF blockers + MTX = anti-TNF treatment plus MTX with or without other DMARDs; MTX = MTX with or without other DMARDs excluding TNF blockers; Control = healthy controls. Levels of significance between groups are shown.
Percentage of patients with immune response, defined as a ≥2-fold increase in antibody level to 6B. For details of groups see legend to Fig. 1. Significant differences are shown.
Percentage of patients with immune response, defined as ≥2-fold increase in antibody level to both 23F and 6B. For details of groups see legend to Fig. 1. Significant differences are shown.
No significant differences or trends could be traced regarding the influences of gender, age, disease duration, disease activity, anti-TNF treatment duration, MTX treatment duration and dosage or prednisolone use and dosage, when testing subjects with a ≥2-fold increase in antibody concentration vs those with an increase of <2-fold and within the different treatment groups. This was found when testing either 23F or 6B alone, or responders to both 23F and 6B. Among healthy controls, those with a ≥2-fold increase in antibody concentration in response to both 23F and 6B were significantly younger than those lacking a response (median 27 vs 38 yr; P = 0.015). This was not statistically significant when testing 23F (P = 0.069) or 6B (P = 0.138) responders vs non-responders alone. The number of subjects with immune responses to both 23F and 6B stratified according to age and treatment group is shown in Table 3. When testing subjects above 60 yr vs those below 60 yr, no statistical differences were found within any of the patient groups.
Immune responses, defined as a ≥2-fold increase in antibody levels for both 23F and 6B in patients grouped according to age and treatment group
| . | TNF blockers without MTX . | . | TNF blockers + MTX . | . | MTX . | . | Controls . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Response . | Yes . | No . | Yes . | No . | Yes . | No . | Yes . | No . | ||||
| >60 | 12 | 8 | 5 | 11 | 4 | 20 | 0 | 3 | ||||
| 50–60 | 11 | 12 | 8 | 10 | 0 | 10 | 2 | 5 | ||||
| 40–50 | 4 | 7 | 1 | 5 | 1 | 1 | 2 | 4 | ||||
| 30–40 | 3 | 3 | 1 | 6 | 0 | 0 | 1 | 8 | ||||
| <30 | 1 | 1 | 1 | 2 | 0 | 1 | 13 | 9 | ||||
| Sum | 31 | 31 | 16 | 34 | 5 | 32 | 18 | 29 | ||||
| . | TNF blockers without MTX . | . | TNF blockers + MTX . | . | MTX . | . | Controls . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Response . | Yes . | No . | Yes . | No . | Yes . | No . | Yes . | No . | ||||
| >60 | 12 | 8 | 5 | 11 | 4 | 20 | 0 | 3 | ||||
| 50–60 | 11 | 12 | 8 | 10 | 0 | 10 | 2 | 5 | ||||
| 40–50 | 4 | 7 | 1 | 5 | 1 | 1 | 2 | 4 | ||||
| 30–40 | 3 | 3 | 1 | 6 | 0 | 0 | 1 | 8 | ||||
| <30 | 1 | 1 | 1 | 2 | 0 | 1 | 13 | 9 | ||||
| Sum | 31 | 31 | 16 | 34 | 5 | 32 | 18 | 29 | ||||
Immune responses, defined as a ≥2-fold increase in antibody levels for both 23F and 6B in patients grouped according to age and treatment group
| . | TNF blockers without MTX . | . | TNF blockers + MTX . | . | MTX . | . | Controls . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Response . | Yes . | No . | Yes . | No . | Yes . | No . | Yes . | No . | ||||
| >60 | 12 | 8 | 5 | 11 | 4 | 20 | 0 | 3 | ||||
| 50–60 | 11 | 12 | 8 | 10 | 0 | 10 | 2 | 5 | ||||
| 40–50 | 4 | 7 | 1 | 5 | 1 | 1 | 2 | 4 | ||||
| 30–40 | 3 | 3 | 1 | 6 | 0 | 0 | 1 | 8 | ||||
| <30 | 1 | 1 | 1 | 2 | 0 | 1 | 13 | 9 | ||||
| Sum | 31 | 31 | 16 | 34 | 5 | 32 | 18 | 29 | ||||
| . | TNF blockers without MTX . | . | TNF blockers + MTX . | . | MTX . | . | Controls . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Response . | Yes . | No . | Yes . | No . | Yes . | No . | Yes . | No . | ||||
| >60 | 12 | 8 | 5 | 11 | 4 | 20 | 0 | 3 | ||||
| 50–60 | 11 | 12 | 8 | 10 | 0 | 10 | 2 | 5 | ||||
| 40–50 | 4 | 7 | 1 | 5 | 1 | 1 | 2 | 4 | ||||
| 30–40 | 3 | 3 | 1 | 6 | 0 | 0 | 1 | 8 | ||||
| <30 | 1 | 1 | 1 | 2 | 0 | 1 | 13 | 9 | ||||
| Sum | 31 | 31 | 16 | 34 | 5 | 32 | 18 | 29 | ||||
Discussion
The major findings in this study are that anti-TNF therapy and prednisolone in low doses do not impair the antibody responses following pneumococcal vaccination, while MTX does reduce the response.
It is well known that immune responses to polysaccharides are lower than responses to protein antigens [20, 41]. Furthermore, precise protective levels have not been established [2, 41, 42]. A twofold increase in the antibody level was chosen as an indicator of immune responsiveness [1–3]. Further uncertainties include variation of individual responses to different pneumococcal polysaccharide antigens [42]. This was also illustrated by our findings (Table 2). Our results show a somewhat lower combined responsiveness compared with a recent study in psoriatic arthritis patients [35], but this may merely reflect the choice of antigens analysed. The rationale for choosing 23F and 6B was that they represent two common serotypes known to be associated with invasive infections that are common among patients with underlying diseases in Sweden [19].
The reduced immune response in the MTX group suggests that reduced responsiveness to exogenous or possibly endogenous antigens may be one of the modes of action of MTX that reduces rheumatoid inflammation. Recent reports of anti-rheumatic effects by B-cell depletion in RA patients suggest that decreased antibody production may decrease the rheumatic inflammation [43, 44]. However, the similar MTX dosages and treatment duration times in patients in the TNF blockers + MTX and MTX groups suggest that drug exposure alone is not satisfactory as the sole explanation, since immune responses were different in the two groups. Instead, individually good clinical RA responses to MTX therapy (53% had low disease activity at vaccination in the MTX group; Table 1) seem to indicate reduced immune responsiveness. Almost all anti-TNF-treated patients had been exposed to MTX prior to anti-TNF therapy, according to guidelines issued by the Swedish Society of Rheumatology. Therefore, patients treated with these remedies represent an RA population with intolerance to or only a partial response to MTX. This is illustrated by the low proportion of patients with low disease activity prior to anti-TNF treatment (6% in the TNF blockers + MTX group; Table 1).
Enhanced immune responses due to anti-TNF treatment are an interesting possibility. There have not been indications of increased total immunoglobulin levels in anti-TNF-treated patients [45], and the findings of increased autoantibody levels in patients undergoing treatment [46–49] could support such a notion. Alternative explanations for the low immune response in the MTX group, such as selection of patients with an inherently good immune response in the other groups, seem less likely. That MTX is the most plausible explanation for the reduced immune response is supported by similar results in reports using other patient groups [32–35].
The analysis of the effect of age on the immune response does not support the notion that high age reduces the likelihood of a positive immune response, at least not among RA patients. An interesting observation in this study is that patients above 60 yr in the group receiving TNF blockers without MTX showed immune responses similar to those of healthy controls below 30 years. However, the analyses of the effect of age at higher ages were not performed due the restricted number of patients. Because of the statistically different mean ages of the control and treatment groups, an influence of age on the immune response cannot be completely ruled out and may be considered a limitation of this study. By contrast, therapy is important for the immune response in RA patients (Table 3).
The prevaccination presence of antibodies to 6B and 23F polysaccharide antigens presumably reflects antibodies acquired during life as a result of pneumococcal infections. Prevaccination antibody levels were particularly high in some subjects in the healthy control group. Also, prevaccination levels of antibodies to pneumococcal polysaccharides are of limited value for selection of patients suitable for vaccination due to variations between different serotypes. The present findings suggest that pneumococcal vaccination should be performed prior to MTX initiation, whereas anti-TNF treatment and a low prednisolone dose do not preclude vaccination during ongoing therapy. Whether vaccinations actually reduce the true incidence of infectious complications in RA patients should be addressed in future trials.

The authors thank nurses Elna Haglund and Eva-Karin Kristoffersson (Department of Rheumatology, Lund University Hospital) for their help with vaccination and collecting the blood samples and Eva Holmström (Section of Microbiology, Institute of Laboratory Medicine, Lund) for performing the antibody assays. The study was supported by grants from the Swedish Rheumatism Association, the Swedish Research Council (grant no. 15092), the Medical Faculty of the University of Lund, Alfred Österlund's Foundation, The Crafoord Foundation, Greta and Johan Kock's foundation, The King Gustaf V Foundation and Lund University Hospital.
The authors have declared no conflicts of interest.
References
Recommendations of the Swedish National Board of Health and Welfare.
CDC Recommendations of the Advisory Committee on Immunization Practices (ACIP).
Go ES, Ballas ZK. Anti-pneumococcal antibody response in normal subjects: a meta-analysis.
Doran MF, Crowson CS, Pond GR, O’Fallon WM, Gabriel SE. Frequency of infections in patients with rheumatoid arthritis compared with controls: a population-based study.
Doran MF, Crowson CS, Pond GR, O’Fallon WM, Gabriel SE. Predictors of infection in rheumatoid arthritis.
Segal BH, Sneller MC. Infectious complications of immunosuppressive therapy in patients with rheumatic disease.
Cunnane G, Doran M, Bresnihan B. Infections and biological therapy in rheumatoid arthritis.
Mitchell DM, Spitz PW, Young DY, Bloch DA, McShaneDJ, Fries JF. Survival, prognosis and causes of death in rheumatoid arthritis.
Wolfe F, Mitchell DM, Sibley JT et al. The mortality of rheumatoid arthritis.
Mohan AK, Cote TR, Siegel JN, Braun MM. Infectious complications of biologic treatment of rheumatoid arthritis.
Keane J, Gershon S, Wise RP et al. Tuberculosis associated with infliximab, a tumour necrosis factor alpha-neutralizing agent.
Lee JH, Slifman NR, Gershon SK, et al. Life-threatening histoplasmosis complicating immunotherapy with tumour necrosis factor alpha antagonists infliximab and etanercept.
Geborek P, Crnkic M, Petersson IF, Saxne T. South Swedish Treatment Group. Etanercept, infliximab and leflunomide in established rheumatoid arthritis: clinical experience using a structured follow up programme in southern Sweden.
Kroesen S, Widmer AF, Tyndall A, Hasler P. Serious bacterial infections in patients with rheumatoid arthritis under anti TNF-alpha therapy.
Ritz MA, Jost R. Severe pneumococcal pneumonia following treatment with infliximab for Cronh's disease.
Chan AT, Cleeve V, Daymond TJ. Necrotising fasciitis in patient receiving infliximab for rheumatoid arthritis.
Baghai M, Osmon DR, Wolk DM, Wold LE, Haidukewych GJ, Matteson EL. Fatal sepsis in a patient with rheumatoid arthritis treated with etanercept.
Henriques Normark B, Kalin M, Örtqrist A et al. Dynamics of penicillin-susceptible clones in invasive pneumococcal disease.
Fedson DS, Musher DM. Pneumococcal vaccine. In: Plotkin SA, Mortimer EA Jr, eds.
Fine MJ, Smith MA, Carson CA et al. Efficacy of pneumococcal vaccination in adults. A meta-analysis of randomised controlled trials.
Cornu C, Yzébe D, Léophonte P, Gaillat J, Boissel JP, Cucherat M. Efficacy of pneumococcal polysaccharide vaccine in immunocompetent adults: a meta-analysis of randomised trials.
Moore RA, Wiffen PJ, Lipsky BA. Are the pneumococcal polysaccharide vaccines effective? Meta-analysis of the prospective trial.
Jackson LA, Neuzil KM, Yu O et al. for the Vaccine Safety Datalink. Effectiveness of pneumococcal polysaccharide vaccine in older adults.
Örtqvist A, Hedlund J, Burman L-A et al. Swedish Pneumococcal Vaccination Study Group. Randomised trial of 23-valent pneumococcal capsular polysaccharide vaccine in prevention of pneumonia in middle-aged and elderly people.
Dear K, Holden J, Andrews R, Tatham D. Vaccine for preventing pneumococcal infection in adults.
Hedlund J, Christenson B, Lundbergh P, Örtqvist Å. Effects of a large-scale intervention with influenza and 23-valent pneumococcal vaccines in elderly people: a 1-year follow-up.
Simberkoff MS, Cross AP, Al-Ibrahim M et al. Efficacy of pneumococcal vaccine in high-risk patients. Results of a Veterans Administration Cooperative Study.
Fry AM, Facklam RR, Whitny CG, Plikaytis BD, Schuchat A. Multistate evaluation of invasive pneumococcal diseases in adults with human immunodeficiency virus infection: serotype and antimicrobial resistance patterns in the United States.
Rubins JB, Alter MD, Loch J, Janoff EN. Determination of antibody responses of eldery adults to all 23 capsular polysaccharides after pneumococcal vaccination.
O’Dell J, Gilg J, Palmer W, Haire C, Klassen L, Moore G. Pneumococcal vaccination: decreased antibody response in rheumatoid arthritis patients on methotrexate.
O’Dell J, Gilg J, Palmer et al. Pneumococcal vaccine in rheumatoid arthritis. Decreased response while on methotrexate.
Elkayam O, Paran D, Caspi D et al. Immunogenicity and safety of pneumococcal vaccination in patients with rheumatoid arthritis or systemic lupus erythematosus.
Elkayam O, Caspi D, Reitblatt T, Charboneau D, Rubins JB. The effect of tumour necrosis factor blockade on the response to pneumococcal vaccination in patients with rheumatoid arthritis and ankylosing spondylitis.
Mease PJ, Ritchlin CT, Martin RW et al. Pneumococcal vaccine response in psoriatic arthritis patients during treatment with etanercept.
Geborek P, Saxne T. Clinical protocol for monitoring of targeted therapies in rheumatoid arthritis.
Van Riel PLCM, van Gestel AM, Scott DL on behalf of the EULAR standing committee for international clinical studies including therapeutic trials.
Van Gestel AM, Prevoo MLL, van't Hof MA, van Rijswijk MH, van den Putte LBA, van Riel PLCM. Development and validation of the European League against Rheumatism response criteria for rheumatoid arthritis. Comparison with preliminary American College and the World Health Organization/International League against Rheumatism criteria.
WHO. Training manual for enzyme linked immunosorbent assay for quantification of Streptococcus pneumoniae serotype specific IgG (PN PS ELISA). Available at: http://www.vaccine.uab.edu/2005_content/WHO9.pdf. Accessed 22 January
Quataert SA, Kirch CS, Wiedl LJ et al. Assignment of weight-based antibody units to a human antipneumococcal standard reference serum, lot 89-S.
Scheifele DW. Using vaccine responses to plumb the immunologic consequences of tumour necrosis factor blockade with etanercept.
Balmer P, North J, Baxter D et al. Measurement and interpretation of pneumococcal IgG levels for clinical management.
Edwards JC, Szczepanski L, Szechinski J et al. Efficacy of B-cell-targeted therapy with rituximab in patients with rheumatoid arthritis.
Edwards JC, Leandro MJ, Cambridge G. B lymphocyte depletion therapy with rituximab in rheumatoid arthritis.
Moreland LW, Bucy RP, Weinblatt ME, Mohler KM, Spencer-Green GT, Chatham WW. Immune function in patients with rheumatoid arthritis treated with etanercept.
Charles PJ, Smeenk RJ, De Jong J, Feldmann M, Maini RN. Assessment of antibodies to double-stranded DNA induced in rheumatoid arthritis patients following treatment with infliximab, a monoclonal antibody to tumour necrosis factor alpha: findings in open-label and randomized placebo-controlled trials.
De Rycke L, Kruithof E, van Damme N et al. Antinuclear antibodies following infliximab treatment in patients with rheumatoid arthritis or spondyloarthropathy.
Bobbio-Pallavicini F, Alpini C, Caporali R, Avalle S, Bugatti S, Montecucco C. Autoantibody profile in rheumatoid arthritis during long-term infliximab treatment.
Author notes
Department of Rheumatology and 1Department of Infectious Diseases, Lund University Hospital and 2Section of Microbiology, Immunology and Glycobiology, Institute of Laboratory Medicine, Lund University, Sweden.
- rheumatoid arthritis
- tumor necrosis factors
- immune response
- antirheumatic drugs, disease-modifying
- etanercept
- antibody formation
- methotrexate
- pneumococcal vaccine
- polysaccharides
- prednisolone
- vaccination
- immunoglobulin g
- antibodies
- infliximab
- pneumococcal vaccine, polyvalent
- pneumococcal 23-valent vaccine
- tumor necrosis factor inhibitors




Comments