-
PDF
- Split View
-
Views
-
Cite
Cite
Veronika K. Jaeger, Hal M. Hoffman, Tom van der Poll, Hugh Tilson, Julia Seibert, Antonio Speziale, Guido Junge, Kristina Franke, Eleni Vritzali, Philip N. Hawkins, Jasmin Kuemmerle-Deschner, Ulrich A. Walker, Safety of vaccinations in patients with cryopyrin-associated periodic syndromes: a prospective registry based study, Rheumatology, Volume 56, Issue 9, September 2017, Pages 1484–1491, https://doi.org/10.1093/rheumatology/kex185
Close - Share Icon Share
Abstract
Pneumococcal, tetanus and influenza vaccinations are recommended for patients with cryopyrin-associated periodic syndromes (CAPS) when treated with immunosuppressive medication. The aim of this publication is to report the safety of pneumococcal and other vaccinations in CAPS patients.
All CAPS patients followed in the β-CONFIDENT (Clinical Outcomes and Safety Registry study of Ilaris patients) registry were analysed if they had received a vaccination. The β-CONFIDENT registry is a global, long-term, prospective, observational registry, capturing and monitoring patients treated with canakinumab.
Sixty-eight CAPS patients had received a total of 159 vaccine injections, 107 injections against influenza, 19 pneumococcal vaccinations, 12 against tetanus/diphtheria antigens and 21 other vaccinations. Fourteen per cent of injections had elicited at least one vaccine reaction. All five vaccine-related serious adverse events were associated with pneumococcal vaccination. Vaccine reactions were observed in 70% of pneumococcal vaccinations, compared with 7% in influenza and 17% in tetanus/diphtheria vaccinations. The odds ratios to react to the pneumococcal vaccines compared with influenza and tetanus/diphtheria vaccines were 31.0 (95% CI: 8, 119) and 10.8 (95% CI: 2, 74). Vaccine reactions after pneumococcal vaccinations were more severe and lasted significantly longer (up to 3 weeks) compared with other vaccinations. In two patients, pneumococcal vaccination also elicited symptoms consistent with systemic inflammation due to CAPS reactivation.
Pneumococcal vaccines, unlike other vaccines, frequently trigger severe local and systemic inflammation in CAPS patients. Clinicians must balance potential benefits of pneumococcal immunization against safety concerns. The 13-valent pneumococcal conjugate vaccine might be favourable over the polysaccharide vaccine in CAPS patients.
Rheumatology key messages
In cryopyrin-associated periodic syndromes patients, pneumococcal vaccinations unlike other vaccinations frequently induced local inflammation.
In cryopyrin-associated periodic syndromes, pneumococcal vaccine reactions were more severe, longer lasting and at times systemic.
Introduction
Cryopyrin-associated periodic syndromes (CAPS), specifically familial cold autoinflammatory syndrome (FCAS), Muckle–Wells syndrome (MWS) and neonatal onset multisystem inflammatory disease (NOMID), are autoinflammatory periodic fever syndromes caused by mutations of the NLRP3 gene that elicit a constitutive activation of the NLRP3 inflammasome and enhance the synthesis of active IL-1β after the detection of danger signals by toll-like receptors (TLRs) [1, 2]. Canakinumab, a human IgG1/k mAb that selectively blocks human IL-1β, has been licensed for the treatment of CAPS [3].
Patients with CAPS, like other patients with immune-related disorders on immunosuppressive treatments, may have an increased susceptibility to pneumococcal pneumonia [4], which even in the general population represents a significant cause of morbidity and mortality [5, 6].
The EULAR and the Advisory Committee on Immunization Practices to the Center for Disease Control and Prevention therefore recommend pneumococcal, tetanus and influenza vaccinations, among other immunizations in patients with periodic fever syndromes treated with immunosuppressive medication [5, 7].
Our early recognition of unusually severe local and/or systemic inflammatory adverse reactions led us to publish information in a case series of seven CAPS patients, consecutively vaccinated with pneumococcal vaccines [8]. Six of the seven patients reported in the case series are also included in this analysis as they were followed in the β-CONFIDENT (Clinical Outcomes and Safety Registry study of Ilaris patients) registry, a long-term prospective observational study of patients treated with canakinumab. Following up on the findings of the case series [8], we prospectively investigated the safety of pneumococcal and other vaccines within the entire β-CONFIDENT registry.
Methods
Study population and design
This study analysed the vaccination safety data of the β-CONFIDENT registry, registered in ClinicalTrials.gov (NCT01213641). Each centre contributing to the β-CONFIDENT registry obtained ethical approval by its local ethics committees or institutional review boards; informed consent according to the Declaration of Helsinki was required from each patient/guardian prior in compliance with local regulations. No additional approval was required for this study analysing the vaccination safety data.
The β-CONFIDENT registry is a global, multicentre, 5 year, prospective, observational registry, capturing long-term safety and effectiveness data of adult and paediatric patients treated with canakinumab [9]. As this was a non-interventional study, patients were treated according to the local prescribing information, and routine medical practice. Data collection was in compliance with Good Medical Practice and Good Pharmacovigilance Practice.
Given the observational nature of this study, the protocol did not mandate any specific schedule of vaccination or follow-up and vaccinations were administered as part of regular medical care according to the independent recommendations of treating physicians.
Collection of vaccination data in the registry started in July 2010 and ended in December 2015. For the purpose of this analysis, patients treated with canakinumab for conditions other than definite CAPS were excluded, as were patients without information of the presence or absence of any vaccination reactions.
Statistical analysis
Categorical variables were calculated as frequencies and percentages and continuous variables were calculated as means with s.d. and medians with interquartile range (IQR). Mann–Whitney U tests were applied for across-group comparisons. Odds ratios and their corresponding 95% CIs were obtained using univariate logistic regression analysis taking the clustering of vaccine injections within one patient into account. All statistical analyses were performed with Stata/IC 13.1 (StataCorp, College Station, TX, USA).
Results
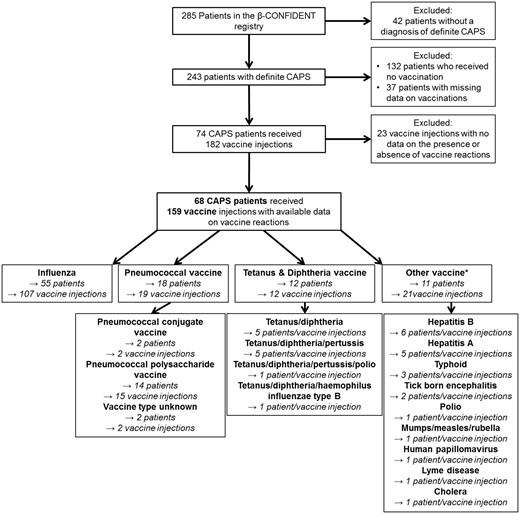
The β-CONFIDENT registry followed 285 patients, 68 of whom fulfilled the inclusion criteria (Fig. 1). The 68 included patients received 159 vaccine injections and were followed in 14 centres in nine countries. The majority of patients (81%) had been vaccinated against influenza, 26% of patients had received a pneumococcal vaccination, 18% of patients had received vaccinations with tetanus and diphtheria antigens and 16% of patients had received at least one vaccine directed against other pathogens (Fig. 1). During the observation period, 43 CAPS patients (63%) had received more than one vaccine injection. Due to the inclusion criteria of the β-CONFIDENT registry, all patients were concomitantly treated with canakinumab at enrolment into the registry. The demographic and disease characteristics of the patients are provided in Table 1.
Demographic and disease characteristics of cryopyrin-associated periodic syndromes patients at their first vaccination
| Characteristics . | Influenza . | Tetanus + diphtheriaa . | Pneumococcus . | Otherb . | ||
|---|---|---|---|---|---|---|
| . | — . | — . | PCV . | PPV . | Unknown . | — . |
| Patients who received a vaccination, n | 55 | 12 | 2 | 14 | 2 | 11 |
| Age | ||||||
| Mean (s.d.), years | 36 (18) | 24 (13) | 31 (30) | 41 (17) | 31 (18) | 26 (16) |
| Median (IQR), years | 37 (18–51) | 23 (16–30) | 31 (10–52) | 47 (24–54) | 31 (18–43) | 25 (15–45) |
| Patients <16 years, n (%) | 9 (16) | 3 (25) | 1 (50) | 1 (7) | 0 (0) | 4 (36) |
| Female sex, n (%) | 30 (55) | 6 (50) | 2 (100) | 10 (71) | 2 (100) | 4 (36) |
| CAPS symptom duration | ||||||
| Mean (s.d.), years | 30 (19) | 19 (12) | 30 (31) | 36 (21) | 25 (26) | 22 (17) |
| Median (IQR), years | 32 (15–44) | 16 (15–23) | 30 (8–51) | 40 (19–54) | 25 (6–43) | 15 (14–28) |
| CAPS phenotype | ||||||
| NOMID, n (%) | 4 (7) | 0 (0) | 1 (50) | 2 (14) | 0 (0) | 1 (10) |
| MWS, n (%) | 34 (62) | 7 (58) | 0 (0) | 11 (79) | 1 (50) | 5 (45) |
| FCAS, n (%) | 17 (31) | 5 (42) | 1 (50) | 1 (7) | 1 (50) | 5 (45) |
| Known NLRP3 mutation, n (%) | 52 (95) | 11 (92) | 2 (100) | 14 (100) | 2 (100) | 11 (100) |
| Characteristics . | Influenza . | Tetanus + diphtheriaa . | Pneumococcus . | Otherb . | ||
|---|---|---|---|---|---|---|
| . | — . | — . | PCV . | PPV . | Unknown . | — . |
| Patients who received a vaccination, n | 55 | 12 | 2 | 14 | 2 | 11 |
| Age | ||||||
| Mean (s.d.), years | 36 (18) | 24 (13) | 31 (30) | 41 (17) | 31 (18) | 26 (16) |
| Median (IQR), years | 37 (18–51) | 23 (16–30) | 31 (10–52) | 47 (24–54) | 31 (18–43) | 25 (15–45) |
| Patients <16 years, n (%) | 9 (16) | 3 (25) | 1 (50) | 1 (7) | 0 (0) | 4 (36) |
| Female sex, n (%) | 30 (55) | 6 (50) | 2 (100) | 10 (71) | 2 (100) | 4 (36) |
| CAPS symptom duration | ||||||
| Mean (s.d.), years | 30 (19) | 19 (12) | 30 (31) | 36 (21) | 25 (26) | 22 (17) |
| Median (IQR), years | 32 (15–44) | 16 (15–23) | 30 (8–51) | 40 (19–54) | 25 (6–43) | 15 (14–28) |
| CAPS phenotype | ||||||
| NOMID, n (%) | 4 (7) | 0 (0) | 1 (50) | 2 (14) | 0 (0) | 1 (10) |
| MWS, n (%) | 34 (62) | 7 (58) | 0 (0) | 11 (79) | 1 (50) | 5 (45) |
| FCAS, n (%) | 17 (31) | 5 (42) | 1 (50) | 1 (7) | 1 (50) | 5 (45) |
| Known NLRP3 mutation, n (%) | 52 (95) | 11 (92) | 2 (100) | 14 (100) | 2 (100) | 11 (100) |
Vaccines containing tetanus and diphtheria antigens: tetanus/diphtheria (five patients), diphtheria/tetanus/pertussis (five patients), diphtheria/tetanus/pertussis/polio (one patient), diphtheria/tetanus/haemophilus influenzae type b (one patient).
Other vaccines: hepatitis B (six patients), hepatitis A (five patients), typhoid (three patients), tick borne encephalitis (two patients), polio (one patient), mumps, measles, rubella (one patient), human papillomavirus (one patient), lyme disease (one patient) and cholera (one patient). FCAS: familial cold autoinflammatory syndrome; MWS: Muckle–Wells syndrome; NLRP3: nucleotide-binding domain, leucine-rich family pyrin domain containing protein 3; NOMID: neonatal onset multisystem inflammatory disease; PCV: pneumococcal conjugate vaccine; PPV: pneumococcal polysaccharide vaccine.
Demographic and disease characteristics of cryopyrin-associated periodic syndromes patients at their first vaccination
| Characteristics . | Influenza . | Tetanus + diphtheriaa . | Pneumococcus . | Otherb . | ||
|---|---|---|---|---|---|---|
| . | — . | — . | PCV . | PPV . | Unknown . | — . |
| Patients who received a vaccination, n | 55 | 12 | 2 | 14 | 2 | 11 |
| Age | ||||||
| Mean (s.d.), years | 36 (18) | 24 (13) | 31 (30) | 41 (17) | 31 (18) | 26 (16) |
| Median (IQR), years | 37 (18–51) | 23 (16–30) | 31 (10–52) | 47 (24–54) | 31 (18–43) | 25 (15–45) |
| Patients <16 years, n (%) | 9 (16) | 3 (25) | 1 (50) | 1 (7) | 0 (0) | 4 (36) |
| Female sex, n (%) | 30 (55) | 6 (50) | 2 (100) | 10 (71) | 2 (100) | 4 (36) |
| CAPS symptom duration | ||||||
| Mean (s.d.), years | 30 (19) | 19 (12) | 30 (31) | 36 (21) | 25 (26) | 22 (17) |
| Median (IQR), years | 32 (15–44) | 16 (15–23) | 30 (8–51) | 40 (19–54) | 25 (6–43) | 15 (14–28) |
| CAPS phenotype | ||||||
| NOMID, n (%) | 4 (7) | 0 (0) | 1 (50) | 2 (14) | 0 (0) | 1 (10) |
| MWS, n (%) | 34 (62) | 7 (58) | 0 (0) | 11 (79) | 1 (50) | 5 (45) |
| FCAS, n (%) | 17 (31) | 5 (42) | 1 (50) | 1 (7) | 1 (50) | 5 (45) |
| Known NLRP3 mutation, n (%) | 52 (95) | 11 (92) | 2 (100) | 14 (100) | 2 (100) | 11 (100) |
| Characteristics . | Influenza . | Tetanus + diphtheriaa . | Pneumococcus . | Otherb . | ||
|---|---|---|---|---|---|---|
| . | — . | — . | PCV . | PPV . | Unknown . | — . |
| Patients who received a vaccination, n | 55 | 12 | 2 | 14 | 2 | 11 |
| Age | ||||||
| Mean (s.d.), years | 36 (18) | 24 (13) | 31 (30) | 41 (17) | 31 (18) | 26 (16) |
| Median (IQR), years | 37 (18–51) | 23 (16–30) | 31 (10–52) | 47 (24–54) | 31 (18–43) | 25 (15–45) |
| Patients <16 years, n (%) | 9 (16) | 3 (25) | 1 (50) | 1 (7) | 0 (0) | 4 (36) |
| Female sex, n (%) | 30 (55) | 6 (50) | 2 (100) | 10 (71) | 2 (100) | 4 (36) |
| CAPS symptom duration | ||||||
| Mean (s.d.), years | 30 (19) | 19 (12) | 30 (31) | 36 (21) | 25 (26) | 22 (17) |
| Median (IQR), years | 32 (15–44) | 16 (15–23) | 30 (8–51) | 40 (19–54) | 25 (6–43) | 15 (14–28) |
| CAPS phenotype | ||||||
| NOMID, n (%) | 4 (7) | 0 (0) | 1 (50) | 2 (14) | 0 (0) | 1 (10) |
| MWS, n (%) | 34 (62) | 7 (58) | 0 (0) | 11 (79) | 1 (50) | 5 (45) |
| FCAS, n (%) | 17 (31) | 5 (42) | 1 (50) | 1 (7) | 1 (50) | 5 (45) |
| Known NLRP3 mutation, n (%) | 52 (95) | 11 (92) | 2 (100) | 14 (100) | 2 (100) | 11 (100) |
Vaccines containing tetanus and diphtheria antigens: tetanus/diphtheria (five patients), diphtheria/tetanus/pertussis (five patients), diphtheria/tetanus/pertussis/polio (one patient), diphtheria/tetanus/haemophilus influenzae type b (one patient).
Other vaccines: hepatitis B (six patients), hepatitis A (five patients), typhoid (three patients), tick borne encephalitis (two patients), polio (one patient), mumps, measles, rubella (one patient), human papillomavirus (one patient), lyme disease (one patient) and cholera (one patient). FCAS: familial cold autoinflammatory syndrome; MWS: Muckle–Wells syndrome; NLRP3: nucleotide-binding domain, leucine-rich family pyrin domain containing protein 3; NOMID: neonatal onset multisystem inflammatory disease; PCV: pneumococcal conjugate vaccine; PPV: pneumococcal polysaccharide vaccine.
Flow chart of patients included and excluded in the analysis
*Five patients received more than one vaccine injection against different pathogens.
Of the total of 159 vaccine injections administered to the 68 CAPS patients, there were 22 injections in 18 patients that elicited at least one vaccine reaction (Table 2). The majority of vaccine reactions (n = 13) occurred in 12 patients receiving pneumococcal vaccines. Twelve of the 15 pneumococcal polysaccharide vaccine (PPV) injections (80%) elicited a vaccine reaction and none of the two patients who were administered a pneumococcal conjugate vaccine (PCV) showed a reaction. In two pneumococcal vaccinations, one of which elicited a reaction, the exact vaccine type was unknown. The high frequency of pneumococcal vaccine reactions contrasted with that of reactions to other vaccine types; only 17 and 7% of the tetanus/diphtheria and influenza vaccinations elicited a vaccine reaction, respectively. The odds ratio to react to any of the pneumococcal vaccines compared with influenza vaccines was 31.0 (95% CI: 8, 119) and compared with tetanus/diphtheria vaccines was 10.8 (95% CI: 2, 74). Considering only PPV reactions, the odds ratio compared with influenza vaccinations was 57.1 (95% CI: 13, 252) and compared with tetanus/diphtheria vaccinations was 12.2 (95% CI: 1, 102).
Canakinumab exposure of cryopyrin-associated periodic syndromes patients and vaccination reactions according to the vaccine type
| Vaccine . | Influenza . | Tetanus + diphtheriaa . | Pneumococcus . | Otherb . | ||
|---|---|---|---|---|---|---|
| reaction characteristics . | — . | — . | PCV . | PPV . | Unknown . | . |
| Injections, n | 107 | 12 | 2 | 15 | 2 | 21 |
| Time since last canakinumab | ||||||
| Mean (s.d.), days | 56 (138) | 88 (123) | 10 (7) | 22 (18) | 37 (28) | 99 (140) |
| Median (IQR), days | 36 (17–50) | 38 (31–59) | 10 (6–14) | 22 (2–41) | 37 (17–57) | 42 (30–79) |
| ≤30 days; n (%) | 44 (41) | 2 (17) | 2 (100) | 10 (67) | 1 (50) | 6 (29) |
| Last canakinumab dose | ||||||
| Mean (s.d.), mg/kg | 2.6 (1.2) | 3.1 (1.2) | 7.9 (8.3) | 2.7 (1.3) | 1.8 (0.1) | 3.2 (1.7) |
| Median (IQR), mg/kg | 2.1 (1.8–2.7) | 2.7 (2.3–4.1) | 7.9 (2.0–13.8) | 2.2 (1.9–2.5) | 1.8 (1.7–1.8) | 2.4 (2.1–4.4) |
| Patients receiving ≤2 mg/kg, n (%) | 43 (41) | 1 (8) | 0 (0) | 4 (27) | 2 (100) | 3 (14) |
| Injections eliciting any vaccine reaction, n (%) | 7 (7) | 2 (17) | 0 (0) | 12 (80) | 1 (50) | 0 (0) |
| Injections eliciting no reaction, n (%) | 100 (93) | 10 (83) | 2 (100) | 3 (20) | 1 (50) | 21 (100) |
| Injections with one reaction featurec, n (%) | 2 (2) | 0 (0) | 0 (0) | 1 (7) | 1 (50) | 0 (0) |
| Injections with two reaction features, n (%) | 3 (3) | 0 (0) | 0 (0) | 1 (7) | 0 (0) | 0 (0) |
| Injections with three reaction features, n (%) | 2 (2) | 2 (17) | 0 (0) | 4 (26) | 0 (0) | 0 (0) |
| Injections with four reaction features, n (%) | 0 (0) | 0 (0) | 0 (0) | 6 (40) | 0 (0) | 0 (0) |
| Fever, n injections (%) | 2 (2) | 0 (0) | 0 (0) | 7 (47) | 0 (0) | 0 (0) |
| Time to fever onset, median (IQR), days | 2 (1–3) | NA | NA | 1 (1–1) | NA | NA |
| Duration of fever, median (IQR), days | 0.5 (0–1) | NA | NA | 17 (9.5–22) | NA | NA |
| Fever maximum, median (IQR), °C | 38.2 | NA | NA | 39.3 (39.0–39.6) | NA | NA |
| Swelling, n injections (%) | 4 (4) | 2 (17) | 0 (0) | 9 (60) | 0 (0) | 0 (0) |
| Time to swelling onset, median (IQR), days | 0 (0–1) | 1 | NA | 1 (0–1) | NA | NA |
| Swelling duration, median (IQR), days | 2 (1––7) | Unknown | NA | 8 (4–22) | NA | NA |
| Erythema, n injections (%) | 4 (4) | 2 (17) | 0 (0) | 11 (73) | 1 (50) | 0 (0) |
| Time to erythema onset, median (IQR), days | 0 (0–0) | 0.5 (0–1) | NA | 1 (0–3) | 0 | NA |
| Erythema duration, median (IQR), days | 1 (1–9) | Unknown | NA | 9.5 (5.5–17) | Unknown | NA |
| Pain, n injections (%) | 3 (3) | 2 (17) | 0 (0) | 12 (80) | 0 (0) | 0 (0) |
| Time to pain onset, median (IQR), days | 1 | 1 | NA | 0 (0–3) | NA | NA |
| Pain duration, median (IQR), days | 7 | Unknown | NA | 9 (4–22) | NA | NA |
| SAE, n patients (%) | 0 (0) | 0 (0) | 0 (0) | 5 (33.3) | 0 (0) | 0 (0) |
| Vaccine . | Influenza . | Tetanus + diphtheriaa . | Pneumococcus . | Otherb . | ||
|---|---|---|---|---|---|---|
| reaction characteristics . | — . | — . | PCV . | PPV . | Unknown . | . |
| Injections, n | 107 | 12 | 2 | 15 | 2 | 21 |
| Time since last canakinumab | ||||||
| Mean (s.d.), days | 56 (138) | 88 (123) | 10 (7) | 22 (18) | 37 (28) | 99 (140) |
| Median (IQR), days | 36 (17–50) | 38 (31–59) | 10 (6–14) | 22 (2–41) | 37 (17–57) | 42 (30–79) |
| ≤30 days; n (%) | 44 (41) | 2 (17) | 2 (100) | 10 (67) | 1 (50) | 6 (29) |
| Last canakinumab dose | ||||||
| Mean (s.d.), mg/kg | 2.6 (1.2) | 3.1 (1.2) | 7.9 (8.3) | 2.7 (1.3) | 1.8 (0.1) | 3.2 (1.7) |
| Median (IQR), mg/kg | 2.1 (1.8–2.7) | 2.7 (2.3–4.1) | 7.9 (2.0–13.8) | 2.2 (1.9–2.5) | 1.8 (1.7–1.8) | 2.4 (2.1–4.4) |
| Patients receiving ≤2 mg/kg, n (%) | 43 (41) | 1 (8) | 0 (0) | 4 (27) | 2 (100) | 3 (14) |
| Injections eliciting any vaccine reaction, n (%) | 7 (7) | 2 (17) | 0 (0) | 12 (80) | 1 (50) | 0 (0) |
| Injections eliciting no reaction, n (%) | 100 (93) | 10 (83) | 2 (100) | 3 (20) | 1 (50) | 21 (100) |
| Injections with one reaction featurec, n (%) | 2 (2) | 0 (0) | 0 (0) | 1 (7) | 1 (50) | 0 (0) |
| Injections with two reaction features, n (%) | 3 (3) | 0 (0) | 0 (0) | 1 (7) | 0 (0) | 0 (0) |
| Injections with three reaction features, n (%) | 2 (2) | 2 (17) | 0 (0) | 4 (26) | 0 (0) | 0 (0) |
| Injections with four reaction features, n (%) | 0 (0) | 0 (0) | 0 (0) | 6 (40) | 0 (0) | 0 (0) |
| Fever, n injections (%) | 2 (2) | 0 (0) | 0 (0) | 7 (47) | 0 (0) | 0 (0) |
| Time to fever onset, median (IQR), days | 2 (1–3) | NA | NA | 1 (1–1) | NA | NA |
| Duration of fever, median (IQR), days | 0.5 (0–1) | NA | NA | 17 (9.5–22) | NA | NA |
| Fever maximum, median (IQR), °C | 38.2 | NA | NA | 39.3 (39.0–39.6) | NA | NA |
| Swelling, n injections (%) | 4 (4) | 2 (17) | 0 (0) | 9 (60) | 0 (0) | 0 (0) |
| Time to swelling onset, median (IQR), days | 0 (0–1) | 1 | NA | 1 (0–1) | NA | NA |
| Swelling duration, median (IQR), days | 2 (1––7) | Unknown | NA | 8 (4–22) | NA | NA |
| Erythema, n injections (%) | 4 (4) | 2 (17) | 0 (0) | 11 (73) | 1 (50) | 0 (0) |
| Time to erythema onset, median (IQR), days | 0 (0–0) | 0.5 (0–1) | NA | 1 (0–3) | 0 | NA |
| Erythema duration, median (IQR), days | 1 (1–9) | Unknown | NA | 9.5 (5.5–17) | Unknown | NA |
| Pain, n injections (%) | 3 (3) | 2 (17) | 0 (0) | 12 (80) | 0 (0) | 0 (0) |
| Time to pain onset, median (IQR), days | 1 | 1 | NA | 0 (0–3) | NA | NA |
| Pain duration, median (IQR), days | 7 | Unknown | NA | 9 (4–22) | NA | NA |
| SAE, n patients (%) | 0 (0) | 0 (0) | 0 (0) | 5 (33.3) | 0 (0) | 0 (0) |
Unless otherwise stated, the numbers and percentages presented are based on vaccine injections, not patients.
Vaccines containing tetanus and diphtheria antigens: tetanus/diphtheria (five patients), diphtheria/tetanus/pertussis (five patients), diphtheria/tetanus/pertussis/polio (one patient), diphtheria/tetanus/haemophilus influenzae type b (one patient).
Other vaccines: hepatitis B (six patients), hepatitis A (five patients), typhoid (three patients), tick borne encephalitis (two patients), polio (one patient), mumps, measles, rubella (one patient), human papillomavirus (one patient), lyme disease (one patient) and cholera (1 patient).
Vaccine reaction features systematically asked for in the registry consist of fever, swelling, erythema and pain. FCAS: familial cold autoinflammatory syndrome; MWS: Muckle–Wells syndrome; NOMID: neonatal onset multisystem inflammatory disease; NA: not applicable; PCV: pneumococcal conjugate vaccine; PPV: pneumococcal polysaccharide vaccine; SAE: serious adverse event.
Canakinumab exposure of cryopyrin-associated periodic syndromes patients and vaccination reactions according to the vaccine type
| Vaccine . | Influenza . | Tetanus + diphtheriaa . | Pneumococcus . | Otherb . | ||
|---|---|---|---|---|---|---|
| reaction characteristics . | — . | — . | PCV . | PPV . | Unknown . | . |
| Injections, n | 107 | 12 | 2 | 15 | 2 | 21 |
| Time since last canakinumab | ||||||
| Mean (s.d.), days | 56 (138) | 88 (123) | 10 (7) | 22 (18) | 37 (28) | 99 (140) |
| Median (IQR), days | 36 (17–50) | 38 (31–59) | 10 (6–14) | 22 (2–41) | 37 (17–57) | 42 (30–79) |
| ≤30 days; n (%) | 44 (41) | 2 (17) | 2 (100) | 10 (67) | 1 (50) | 6 (29) |
| Last canakinumab dose | ||||||
| Mean (s.d.), mg/kg | 2.6 (1.2) | 3.1 (1.2) | 7.9 (8.3) | 2.7 (1.3) | 1.8 (0.1) | 3.2 (1.7) |
| Median (IQR), mg/kg | 2.1 (1.8–2.7) | 2.7 (2.3–4.1) | 7.9 (2.0–13.8) | 2.2 (1.9–2.5) | 1.8 (1.7–1.8) | 2.4 (2.1–4.4) |
| Patients receiving ≤2 mg/kg, n (%) | 43 (41) | 1 (8) | 0 (0) | 4 (27) | 2 (100) | 3 (14) |
| Injections eliciting any vaccine reaction, n (%) | 7 (7) | 2 (17) | 0 (0) | 12 (80) | 1 (50) | 0 (0) |
| Injections eliciting no reaction, n (%) | 100 (93) | 10 (83) | 2 (100) | 3 (20) | 1 (50) | 21 (100) |
| Injections with one reaction featurec, n (%) | 2 (2) | 0 (0) | 0 (0) | 1 (7) | 1 (50) | 0 (0) |
| Injections with two reaction features, n (%) | 3 (3) | 0 (0) | 0 (0) | 1 (7) | 0 (0) | 0 (0) |
| Injections with three reaction features, n (%) | 2 (2) | 2 (17) | 0 (0) | 4 (26) | 0 (0) | 0 (0) |
| Injections with four reaction features, n (%) | 0 (0) | 0 (0) | 0 (0) | 6 (40) | 0 (0) | 0 (0) |
| Fever, n injections (%) | 2 (2) | 0 (0) | 0 (0) | 7 (47) | 0 (0) | 0 (0) |
| Time to fever onset, median (IQR), days | 2 (1–3) | NA | NA | 1 (1–1) | NA | NA |
| Duration of fever, median (IQR), days | 0.5 (0–1) | NA | NA | 17 (9.5–22) | NA | NA |
| Fever maximum, median (IQR), °C | 38.2 | NA | NA | 39.3 (39.0–39.6) | NA | NA |
| Swelling, n injections (%) | 4 (4) | 2 (17) | 0 (0) | 9 (60) | 0 (0) | 0 (0) |
| Time to swelling onset, median (IQR), days | 0 (0–1) | 1 | NA | 1 (0–1) | NA | NA |
| Swelling duration, median (IQR), days | 2 (1––7) | Unknown | NA | 8 (4–22) | NA | NA |
| Erythema, n injections (%) | 4 (4) | 2 (17) | 0 (0) | 11 (73) | 1 (50) | 0 (0) |
| Time to erythema onset, median (IQR), days | 0 (0–0) | 0.5 (0–1) | NA | 1 (0–3) | 0 | NA |
| Erythema duration, median (IQR), days | 1 (1–9) | Unknown | NA | 9.5 (5.5–17) | Unknown | NA |
| Pain, n injections (%) | 3 (3) | 2 (17) | 0 (0) | 12 (80) | 0 (0) | 0 (0) |
| Time to pain onset, median (IQR), days | 1 | 1 | NA | 0 (0–3) | NA | NA |
| Pain duration, median (IQR), days | 7 | Unknown | NA | 9 (4–22) | NA | NA |
| SAE, n patients (%) | 0 (0) | 0 (0) | 0 (0) | 5 (33.3) | 0 (0) | 0 (0) |
| Vaccine . | Influenza . | Tetanus + diphtheriaa . | Pneumococcus . | Otherb . | ||
|---|---|---|---|---|---|---|
| reaction characteristics . | — . | — . | PCV . | PPV . | Unknown . | . |
| Injections, n | 107 | 12 | 2 | 15 | 2 | 21 |
| Time since last canakinumab | ||||||
| Mean (s.d.), days | 56 (138) | 88 (123) | 10 (7) | 22 (18) | 37 (28) | 99 (140) |
| Median (IQR), days | 36 (17–50) | 38 (31–59) | 10 (6–14) | 22 (2–41) | 37 (17–57) | 42 (30–79) |
| ≤30 days; n (%) | 44 (41) | 2 (17) | 2 (100) | 10 (67) | 1 (50) | 6 (29) |
| Last canakinumab dose | ||||||
| Mean (s.d.), mg/kg | 2.6 (1.2) | 3.1 (1.2) | 7.9 (8.3) | 2.7 (1.3) | 1.8 (0.1) | 3.2 (1.7) |
| Median (IQR), mg/kg | 2.1 (1.8–2.7) | 2.7 (2.3–4.1) | 7.9 (2.0–13.8) | 2.2 (1.9–2.5) | 1.8 (1.7–1.8) | 2.4 (2.1–4.4) |
| Patients receiving ≤2 mg/kg, n (%) | 43 (41) | 1 (8) | 0 (0) | 4 (27) | 2 (100) | 3 (14) |
| Injections eliciting any vaccine reaction, n (%) | 7 (7) | 2 (17) | 0 (0) | 12 (80) | 1 (50) | 0 (0) |
| Injections eliciting no reaction, n (%) | 100 (93) | 10 (83) | 2 (100) | 3 (20) | 1 (50) | 21 (100) |
| Injections with one reaction featurec, n (%) | 2 (2) | 0 (0) | 0 (0) | 1 (7) | 1 (50) | 0 (0) |
| Injections with two reaction features, n (%) | 3 (3) | 0 (0) | 0 (0) | 1 (7) | 0 (0) | 0 (0) |
| Injections with three reaction features, n (%) | 2 (2) | 2 (17) | 0 (0) | 4 (26) | 0 (0) | 0 (0) |
| Injections with four reaction features, n (%) | 0 (0) | 0 (0) | 0 (0) | 6 (40) | 0 (0) | 0 (0) |
| Fever, n injections (%) | 2 (2) | 0 (0) | 0 (0) | 7 (47) | 0 (0) | 0 (0) |
| Time to fever onset, median (IQR), days | 2 (1–3) | NA | NA | 1 (1–1) | NA | NA |
| Duration of fever, median (IQR), days | 0.5 (0–1) | NA | NA | 17 (9.5–22) | NA | NA |
| Fever maximum, median (IQR), °C | 38.2 | NA | NA | 39.3 (39.0–39.6) | NA | NA |
| Swelling, n injections (%) | 4 (4) | 2 (17) | 0 (0) | 9 (60) | 0 (0) | 0 (0) |
| Time to swelling onset, median (IQR), days | 0 (0–1) | 1 | NA | 1 (0–1) | NA | NA |
| Swelling duration, median (IQR), days | 2 (1––7) | Unknown | NA | 8 (4–22) | NA | NA |
| Erythema, n injections (%) | 4 (4) | 2 (17) | 0 (0) | 11 (73) | 1 (50) | 0 (0) |
| Time to erythema onset, median (IQR), days | 0 (0–0) | 0.5 (0–1) | NA | 1 (0–3) | 0 | NA |
| Erythema duration, median (IQR), days | 1 (1–9) | Unknown | NA | 9.5 (5.5–17) | Unknown | NA |
| Pain, n injections (%) | 3 (3) | 2 (17) | 0 (0) | 12 (80) | 0 (0) | 0 (0) |
| Time to pain onset, median (IQR), days | 1 | 1 | NA | 0 (0–3) | NA | NA |
| Pain duration, median (IQR), days | 7 | Unknown | NA | 9 (4–22) | NA | NA |
| SAE, n patients (%) | 0 (0) | 0 (0) | 0 (0) | 5 (33.3) | 0 (0) | 0 (0) |
Unless otherwise stated, the numbers and percentages presented are based on vaccine injections, not patients.
Vaccines containing tetanus and diphtheria antigens: tetanus/diphtheria (five patients), diphtheria/tetanus/pertussis (five patients), diphtheria/tetanus/pertussis/polio (one patient), diphtheria/tetanus/haemophilus influenzae type b (one patient).
Other vaccines: hepatitis B (six patients), hepatitis A (five patients), typhoid (three patients), tick borne encephalitis (two patients), polio (one patient), mumps, measles, rubella (one patient), human papillomavirus (one patient), lyme disease (one patient) and cholera (1 patient).
Vaccine reaction features systematically asked for in the registry consist of fever, swelling, erythema and pain. FCAS: familial cold autoinflammatory syndrome; MWS: Muckle–Wells syndrome; NOMID: neonatal onset multisystem inflammatory disease; NA: not applicable; PCV: pneumococcal conjugate vaccine; PPV: pneumococcal polysaccharide vaccine; SAE: serious adverse event.
The severity of the vaccine reactions, as assessed by the number and intensity of different inflammatory symptoms (fever, swelling, erythema and pain) was also increased after PPV vaccination compared with other vaccines (Table 2). Fever was elicited by almost half of all PPV injections. All symptoms after pneumococcal vaccination were observed very rapidly, usually within hours. Unlike the symptoms observed after influenza, tetanus or diphtheria vaccination, which resolved rapidly, the symptoms related to PPV were much more prolonged and in some cases lasted >3 weeks.
In two MWS patients, PPV exposure was also associated with symptoms attributable to CAPS reactivation. One patient developed meningitis and the CRP was reported to be elevated one day after PPV injection. The events resolved after a period of 10–18 days.
During the entire observation period, there were a total of five patients experiencing vaccine-related serious adverse events (SAEs). Four events were observed in MWS patients and one in a NOMID patient; all were observed after PPV injections. The SAEs required hospital treatment in three cases. In one patient, a hospital consultation was necessary due to fever and local inflammation 1 day after PPV. Hospitalization was required in two patients, 4 days after PPV due to meningitis and cellulitis at the injected arm in one patient and 7 days after PPV due to progressive cellulitis at the injected arm in another patient. A fourth patient also developed local inflammation immediately after the injection. Over the next 3 days, this patient worsened and developed a swollen, hot erythematous arm, as well as nausea, headache and fever. A fifth patient developed local inflammation and fever 1 day after the PPV injection.
In all five patients, the vaccine-related SAEs resolved after a period of 10–28 days.
Two patients had been vaccinated twice with pneumococcal vaccine. One patient first received PCV followed by PPV 2 months later and did not experience any vaccine reactions. The second patient received two PPV vaccines 1.8 years apart. This patient experienced only pain at the first vaccination, but developed pain, fever and erythema that lasted for 12 days after the second PPV injection.
Nine of the 12 patients who had reacted to pneumococcal vaccines had been previously (n = 7) or subsequently (n = 5) vaccinated with other vaccines (18 influenza vaccine injections and 2 vaccinations against tetanus/diphtheria). In these nine patients, none of the other vaccine injections had elicited a vaccine reaction.
Comparing the pneumococcal vaccine reactions among the different CAPS phenotypes, patients with NOMID had a higher nominal proportion of different injection site symptoms than patients with MWS or FCAS and a higher incidence of fever (Table 3). Patients who reacted to pneumococcal vaccines also tended to be younger than those who did not react. The former also had a slightly shorter time since the last administration of canakinumab, but had received lower canakinumab doses (Table 4). There was also no association between the time from the last canakinumab administration, or the canakinumab dose and the number of reaction symptoms (P = 0.57, P = 0.17, respectively).
Vaccine reactions after pneumococcal vaccine injections, stratified by cryopyrin-associated periodic syndromes phenotype
| Vaccine reaction characteristics . | NOMID . | MWS . | FCAS . |
|---|---|---|---|
| Pneumococcal vaccinations, n | 3 | 13 | 3 |
| Injections eliciting a vaccine reaction, n (%) | 2 (67) | 10 (77) | 1 (33) |
| Injections eliciting no reaction, n (%) | 1 (33) | 3 (23) | 2 (67) |
| Injections with one reaction feature, n (%) | 0 (0) | 1 (8) | 1 (30) |
| Injections with two reaction features, n (%) | 0 (0) | 1 (8) | 0 (0) |
| Injections with three reaction features, n (%) | 0 (0) | 4 (31) | 0 (0) |
| Injections with four reaction features, n (%) | 2 (67) | 4 (30) | 0 (0) |
| Fever, n (%) | 2 (67) | 5 (38) | 0 (0) |
| Time to fever onset, median (IQR), days | Unknown | 1 (1–1) | NA |
| Duration of fever, median (IQR), days | Unknown | 17 (10–22) | NA |
| Swelling, n (%) | 2 (67) | 7 (54) | 0 (0) |
| Time to swelling onset, median (IQR), days | 1 (1–1) | 1 (0–1) | NA |
| Duration of swelling, median (IQR), days | Unknown | 8 (4–22) | NA |
| Erythema, n (%) | 2 (67) | 9 (69) | 1 (33) |
| Time to erythema onset, median (IQR), days | 0.5 (0–1) | 1 (0–1) | 0 (0) |
| Duration of erythema, median (IQR), days | Unknown | 10 (6–17) | Unknown |
| Pain, n (%) | 2 (67) | 10 (77) | 0 (0) |
| Time to pain onset, median (IQR), days | 0 (0–0) | 0 (0–1) | NA |
| Duration of pain, median (IQR), days | Unknown | 9 (6–17) | NA |
| SAE; n patients (%) | 1 (33) | 4 (31) | 0 (0) |
| Vaccine reaction characteristics . | NOMID . | MWS . | FCAS . |
|---|---|---|---|
| Pneumococcal vaccinations, n | 3 | 13 | 3 |
| Injections eliciting a vaccine reaction, n (%) | 2 (67) | 10 (77) | 1 (33) |
| Injections eliciting no reaction, n (%) | 1 (33) | 3 (23) | 2 (67) |
| Injections with one reaction feature, n (%) | 0 (0) | 1 (8) | 1 (30) |
| Injections with two reaction features, n (%) | 0 (0) | 1 (8) | 0 (0) |
| Injections with three reaction features, n (%) | 0 (0) | 4 (31) | 0 (0) |
| Injections with four reaction features, n (%) | 2 (67) | 4 (30) | 0 (0) |
| Fever, n (%) | 2 (67) | 5 (38) | 0 (0) |
| Time to fever onset, median (IQR), days | Unknown | 1 (1–1) | NA |
| Duration of fever, median (IQR), days | Unknown | 17 (10–22) | NA |
| Swelling, n (%) | 2 (67) | 7 (54) | 0 (0) |
| Time to swelling onset, median (IQR), days | 1 (1–1) | 1 (0–1) | NA |
| Duration of swelling, median (IQR), days | Unknown | 8 (4–22) | NA |
| Erythema, n (%) | 2 (67) | 9 (69) | 1 (33) |
| Time to erythema onset, median (IQR), days | 0.5 (0–1) | 1 (0–1) | 0 (0) |
| Duration of erythema, median (IQR), days | Unknown | 10 (6–17) | Unknown |
| Pain, n (%) | 2 (67) | 10 (77) | 0 (0) |
| Time to pain onset, median (IQR), days | 0 (0–0) | 0 (0–1) | NA |
| Duration of pain, median (IQR), days | Unknown | 9 (6–17) | NA |
| SAE; n patients (%) | 1 (33) | 4 (31) | 0 (0) |
Unless otherwise stated, the numbers and percentages presented are based on vaccine injections, not patients. FCAS: familial cold autoinflammatory syndrome; MWS: Muckle–Wells syndrome; NA: not applicable; NOMID: neonatal onset multisystem inflammatory disease; SAE: serious adverse event.
Vaccine reactions after pneumococcal vaccine injections, stratified by cryopyrin-associated periodic syndromes phenotype
| Vaccine reaction characteristics . | NOMID . | MWS . | FCAS . |
|---|---|---|---|
| Pneumococcal vaccinations, n | 3 | 13 | 3 |
| Injections eliciting a vaccine reaction, n (%) | 2 (67) | 10 (77) | 1 (33) |
| Injections eliciting no reaction, n (%) | 1 (33) | 3 (23) | 2 (67) |
| Injections with one reaction feature, n (%) | 0 (0) | 1 (8) | 1 (30) |
| Injections with two reaction features, n (%) | 0 (0) | 1 (8) | 0 (0) |
| Injections with three reaction features, n (%) | 0 (0) | 4 (31) | 0 (0) |
| Injections with four reaction features, n (%) | 2 (67) | 4 (30) | 0 (0) |
| Fever, n (%) | 2 (67) | 5 (38) | 0 (0) |
| Time to fever onset, median (IQR), days | Unknown | 1 (1–1) | NA |
| Duration of fever, median (IQR), days | Unknown | 17 (10–22) | NA |
| Swelling, n (%) | 2 (67) | 7 (54) | 0 (0) |
| Time to swelling onset, median (IQR), days | 1 (1–1) | 1 (0–1) | NA |
| Duration of swelling, median (IQR), days | Unknown | 8 (4–22) | NA |
| Erythema, n (%) | 2 (67) | 9 (69) | 1 (33) |
| Time to erythema onset, median (IQR), days | 0.5 (0–1) | 1 (0–1) | 0 (0) |
| Duration of erythema, median (IQR), days | Unknown | 10 (6–17) | Unknown |
| Pain, n (%) | 2 (67) | 10 (77) | 0 (0) |
| Time to pain onset, median (IQR), days | 0 (0–0) | 0 (0–1) | NA |
| Duration of pain, median (IQR), days | Unknown | 9 (6–17) | NA |
| SAE; n patients (%) | 1 (33) | 4 (31) | 0 (0) |
| Vaccine reaction characteristics . | NOMID . | MWS . | FCAS . |
|---|---|---|---|
| Pneumococcal vaccinations, n | 3 | 13 | 3 |
| Injections eliciting a vaccine reaction, n (%) | 2 (67) | 10 (77) | 1 (33) |
| Injections eliciting no reaction, n (%) | 1 (33) | 3 (23) | 2 (67) |
| Injections with one reaction feature, n (%) | 0 (0) | 1 (8) | 1 (30) |
| Injections with two reaction features, n (%) | 0 (0) | 1 (8) | 0 (0) |
| Injections with three reaction features, n (%) | 0 (0) | 4 (31) | 0 (0) |
| Injections with four reaction features, n (%) | 2 (67) | 4 (30) | 0 (0) |
| Fever, n (%) | 2 (67) | 5 (38) | 0 (0) |
| Time to fever onset, median (IQR), days | Unknown | 1 (1–1) | NA |
| Duration of fever, median (IQR), days | Unknown | 17 (10–22) | NA |
| Swelling, n (%) | 2 (67) | 7 (54) | 0 (0) |
| Time to swelling onset, median (IQR), days | 1 (1–1) | 1 (0–1) | NA |
| Duration of swelling, median (IQR), days | Unknown | 8 (4–22) | NA |
| Erythema, n (%) | 2 (67) | 9 (69) | 1 (33) |
| Time to erythema onset, median (IQR), days | 0.5 (0–1) | 1 (0–1) | 0 (0) |
| Duration of erythema, median (IQR), days | Unknown | 10 (6–17) | Unknown |
| Pain, n (%) | 2 (67) | 10 (77) | 0 (0) |
| Time to pain onset, median (IQR), days | 0 (0–0) | 0 (0–1) | NA |
| Duration of pain, median (IQR), days | Unknown | 9 (6–17) | NA |
| SAE; n patients (%) | 1 (33) | 4 (31) | 0 (0) |
Unless otherwise stated, the numbers and percentages presented are based on vaccine injections, not patients. FCAS: familial cold autoinflammatory syndrome; MWS: Muckle–Wells syndrome; NA: not applicable; NOMID: neonatal onset multisystem inflammatory disease; SAE: serious adverse event.
Comparison of demographic and treatment characteristics of cryopyrin-associated periodic syndromes patients receiving pneumococcal vaccine injections
| Characteristics . | No reaction . | Any reaction . |
|---|---|---|
| Pneumococcal vaccine injections, n (%) | 6 | 13 |
| Age | ||
| Mean (s.d.), years | 46 (19) | 36 (16) |
| Median (IQR), years | 52 (43–54) | 42 (20–50) |
| Patients <16 years, n (%) | 1 (17) | 1 (8) |
| Female sex, n vaccine injections (%) | 6 (100) | 8 (62) |
| CAPS symptom duration | ||
| Mean (s.d.), years | 45 (20) | 29 (19) |
| Median (IQR), years | 52 (43–54) | 26 (15–42) |
| NLRP3 mutation, n (%) | 6 (100) | 13 (100) |
| Time since last canakinumab | ||
| Mean (s.d.), days | 29 (24) | 19 (16) |
| Median (IQR), days | 26 (6–56) | 18 (2–37) |
| ≤30 days, n (%) | 3 (50) | 10 (77) |
| >30 days, n (%) | 3 (50) | 3 (23) |
| Last canakinumab dose | ||
| Mean (s.d.), mg/kg | 4.0 (4.8) | 2.7 (1.4) |
| Median (IQR), mg/kg | 2.0 (2.0–2.3) | 2.2 (1.9–2.5) |
| Patients receiving ≤2 mg/kg, n (%) | 1 (17) | 5 (38) |
| Patients receiving >2 mg/kg, n (%) | 3 (83) | 8 (62) |
| Characteristics . | No reaction . | Any reaction . |
|---|---|---|
| Pneumococcal vaccine injections, n (%) | 6 | 13 |
| Age | ||
| Mean (s.d.), years | 46 (19) | 36 (16) |
| Median (IQR), years | 52 (43–54) | 42 (20–50) |
| Patients <16 years, n (%) | 1 (17) | 1 (8) |
| Female sex, n vaccine injections (%) | 6 (100) | 8 (62) |
| CAPS symptom duration | ||
| Mean (s.d.), years | 45 (20) | 29 (19) |
| Median (IQR), years | 52 (43–54) | 26 (15–42) |
| NLRP3 mutation, n (%) | 6 (100) | 13 (100) |
| Time since last canakinumab | ||
| Mean (s.d.), days | 29 (24) | 19 (16) |
| Median (IQR), days | 26 (6–56) | 18 (2–37) |
| ≤30 days, n (%) | 3 (50) | 10 (77) |
| >30 days, n (%) | 3 (50) | 3 (23) |
| Last canakinumab dose | ||
| Mean (s.d.), mg/kg | 4.0 (4.8) | 2.7 (1.4) |
| Median (IQR), mg/kg | 2.0 (2.0–2.3) | 2.2 (1.9–2.5) |
| Patients receiving ≤2 mg/kg, n (%) | 1 (17) | 5 (38) |
| Patients receiving >2 mg/kg, n (%) | 3 (83) | 8 (62) |
The numbers and percentages presented are based on vaccine injections, not patients. NLRP3: nucleotide-binding domain, leucine-rich family pyrin domain containing 3.
Comparison of demographic and treatment characteristics of cryopyrin-associated periodic syndromes patients receiving pneumococcal vaccine injections
| Characteristics . | No reaction . | Any reaction . |
|---|---|---|
| Pneumococcal vaccine injections, n (%) | 6 | 13 |
| Age | ||
| Mean (s.d.), years | 46 (19) | 36 (16) |
| Median (IQR), years | 52 (43–54) | 42 (20–50) |
| Patients <16 years, n (%) | 1 (17) | 1 (8) |
| Female sex, n vaccine injections (%) | 6 (100) | 8 (62) |
| CAPS symptom duration | ||
| Mean (s.d.), years | 45 (20) | 29 (19) |
| Median (IQR), years | 52 (43–54) | 26 (15–42) |
| NLRP3 mutation, n (%) | 6 (100) | 13 (100) |
| Time since last canakinumab | ||
| Mean (s.d.), days | 29 (24) | 19 (16) |
| Median (IQR), days | 26 (6–56) | 18 (2–37) |
| ≤30 days, n (%) | 3 (50) | 10 (77) |
| >30 days, n (%) | 3 (50) | 3 (23) |
| Last canakinumab dose | ||
| Mean (s.d.), mg/kg | 4.0 (4.8) | 2.7 (1.4) |
| Median (IQR), mg/kg | 2.0 (2.0–2.3) | 2.2 (1.9–2.5) |
| Patients receiving ≤2 mg/kg, n (%) | 1 (17) | 5 (38) |
| Patients receiving >2 mg/kg, n (%) | 3 (83) | 8 (62) |
| Characteristics . | No reaction . | Any reaction . |
|---|---|---|
| Pneumococcal vaccine injections, n (%) | 6 | 13 |
| Age | ||
| Mean (s.d.), years | 46 (19) | 36 (16) |
| Median (IQR), years | 52 (43–54) | 42 (20–50) |
| Patients <16 years, n (%) | 1 (17) | 1 (8) |
| Female sex, n vaccine injections (%) | 6 (100) | 8 (62) |
| CAPS symptom duration | ||
| Mean (s.d.), years | 45 (20) | 29 (19) |
| Median (IQR), years | 52 (43–54) | 26 (15–42) |
| NLRP3 mutation, n (%) | 6 (100) | 13 (100) |
| Time since last canakinumab | ||
| Mean (s.d.), days | 29 (24) | 19 (16) |
| Median (IQR), days | 26 (6–56) | 18 (2–37) |
| ≤30 days, n (%) | 3 (50) | 10 (77) |
| >30 days, n (%) | 3 (50) | 3 (23) |
| Last canakinumab dose | ||
| Mean (s.d.), mg/kg | 4.0 (4.8) | 2.7 (1.4) |
| Median (IQR), mg/kg | 2.0 (2.0–2.3) | 2.2 (1.9–2.5) |
| Patients receiving ≤2 mg/kg, n (%) | 1 (17) | 5 (38) |
| Patients receiving >2 mg/kg, n (%) | 3 (83) | 8 (62) |
The numbers and percentages presented are based on vaccine injections, not patients. NLRP3: nucleotide-binding domain, leucine-rich family pyrin domain containing 3.
Antibody titre measurements following vaccination were recorded in a total of four patients, all of whom had received PPV. In these four patients, PPV had resulted in titres considered to be protective.
Discussion
This prospective observational study demonstrates that in CAPS patients, pneumococcal vaccines are associated with adverse events that are more frequent, more severe and longer lasting than comparator vaccines such as tetanus, diphtheria and influenza. Pneumococcal vaccination was also associated with a high rate of serious events. The events associated in this study with pneumococcal vaccinations also appear more frequent and more intense than those previously reported after pneumococcal immunization in healthy persons [10] and in patients with other rheumatic diseases [11]. Of note, there was a strikingly higher incidence of serious post-pneumococcal vaccine reactions, also appeared more severe than those previously reported in healthy persons and in patients with other rheumatic diseases [10].
Some cases in this study also suggested that pneumococcal vaccines can trigger systemic inflammation and even CAPS flares. Our early recognition of these findings led us to publish information about six patients from the registry and an additional patient not treated with canakinumab [8]. The present study confirms, extends and puts into perspective the findings from the previous case series. One hypothesis that could explain the adverse events following pneumococcal vaccination is that pneumococcal antigens contain TLR2 and TLR4 ligands that trigger the rapid onset and systemic symptoms in patients who are genetically prone to inflammasome overactivation, i.e. in CAPS patients [8]. The observation that patients with the more severe CAPS phenotype (NOMID) appeared to have more frequent and more severe events than CAPS patients with the less severe phenotype (FCAS) also supports a role of inflammasome hyperactivation in this adverse reaction. PPV commonly utilizes purified polysaccharides from 23 pneumococcal serotypes; immunity is induced primarily through stimulation of IgM-secreting B cells without the assistance of T cells. Since there is no immunoglobulin type switch, the PPV vaccine does not elicit IgA antibodies and mucosal immunity; immunization is not life-long [12]. PCV contains capsular polysaccharides covalently bound to diphtheria toxoid, but employs antigens of fewer pneumococcal serotypes than PPV. In contrast to PPV, PCV elicits long lasting T-helper cell-dependent immune responses, immunoglobulin type switching, mucosal immunity and immunological memory [12]. Interestingly, all of the adverse events described in this study after pneumococcal vaccinations were associated with PPV, though numbers are too small to warrant any conclusion about a potential difference between the two. Both vaccines, however, contain TLR2 and 4 ligands and vaccine reactions to PCV have been also observed in a 24-year-old woman with FCAS not followed in this registry (H.M.H. unpublished observation) as well as another CAPS case [8], suggesting that the inflammatory reaction is not restricted to the PPV vaccine subtype.
Moreover, familial Mediterranean fever is a condition associated with increased inflammasome activation [13]. The β-CONFIDENT registry also followed a boy with familial Mediterranean fever. This boy (not included in this analysis of the registry) was vaccinated with PPV at the age of 13 years. Within <1 day, the boy experienced severe pain and massive swelling at the injection site. These symptoms lasted for 7 days. Further support for the permissive role of the inflammasome for the pneumococcal vaccine reactions stems from similar events reported in patient with Behçet’s disease [14], a condition also associated with aberrant inflammasome activation [13, 15].
It is intriguing that not all patients receiving pneumococcal vaccines in the β-CONFIDENT registry appear to have reacted to pneumococcal vaccines. We cannot fully rule out recall deficits or incomplete data capture as a possible explanation. On the other hand, canakinumab may be expected to mitigate symptoms by counteracting an enhanced IL-1β activation. Nevertheless we failed to identify a protective effect of canakinumab in our CAPS patients as there was no association between the presence or severity of the vaccine reactions with canakinumab dose or timing. Local vaccine reactions could, however, also be explained by insufficient canakinumab concentrations. The lack of a protective effect of canakinumab may, also result from the involvement of proinflammatory pathways other than IL-1β, as the activated inflammasome can also activate preforms of IL-18, induce inflammatory cell death known as pyroptosis and enhance eicosanoid production [13, 14].
The risk of adverse vaccine reactions must be clearly weighed against the risk of pneumococcal infections. Mice with dysfunctional NLRP3 signalling are more susceptible to pneumococcal pneumonia [4, 14], providing an argument in favour of continued vaccination. In the absence of more robust data it is unclear if both types of pneumococcal vaccines elicit inflammatory symptoms to a similar degree. The authors tend to favour the 13-valent PCV over PPV in CAPS patients, also for the immunological advantages of PCV discussed above.
The NLRP3 inflammasome plays an important role in the generation of pneumococcal antibodies following vaccination and is also required for the effects of aluminium and other vaccine adjuvants raising the possibility of an impaired vaccine efficacy [15–17]. We did not detect an impaired antibody production in the few patients treated with canakinumab that had pneumococcal antibody titre measurements. Future research should consider systematic measurements of antibody titres and could also investigate if pneumococcal vaccine side effects could be mitigated by employing reduced antigen doses, or by concomitantly administering anti-inflammatory medications such as cyclooxygenase inhibitors and glucocorticosteroids [13, 18].
In conclusion, pneumococcal vaccines, unlike other vaccines, trigger severe local and systemic inflammation in CAPS. Clinicians must weigh potential benefits of pneumococcal immunization against safety concerns.
Acknowledgements
The authors thank the patients and investigators who participated in this study.
Funding: This work was supported by Novartis Pharma AG, Basel, Switzerland.
Disclosure statement: E.V., J.G. and J.S. are employees of Novartis Pharma, Switzerland. U.A.W., P.N.H., H.T., T.vdP. and H.H. served in the steering committee of the β-CONFIDENT registry and in this function received consulting fees from Novartis, the company that produces canakinumab. A.S. is an employee and shareholder at Novartis. J.K.D. performed clinical studies with Novartis and received honoraria from Novartis and SOBI. K.F. is a full-time employee of QuintilesIMS, which provides research and consulting services to the biopharmaceutical industry and was contracted by Novartis to support this Registry. The other author has declared no conflicts of interest.
References
- influenza
- immunosuppressive agents
- inflammation
- diphtheria vaccine
- antigens
- diphtheria-tetanus vaccine
- influenza vaccines
- pneumococcal vaccine
- polysaccharides
- precipitating factors
- safety
- vaccination
- vaccines
- vaccines, conjugate
- diphtheria
- tetanus
- treatment outcome
- adverse event
- canakinumab
- cryopyrin-associated periodic syndromes
- patient monitoring
- vaccine safety



Comments