-
PDF
- Split View
-
Views
-
Cite
Cite
William Tillett, Rachel Charlton, Alison Nightingale, Julia Snowball, Amelia Green, Catherine Smith, Gavin Shaddick, Neil McHugh, Interval between onset of psoriasis and psoriatic arthritis comparing the UK Clinical Practice Research Datalink with a hospital-based cohort, Rheumatology, Volume 56, Issue 12, December 2017, Pages 2109–2113, https://doi.org/10.1093/rheumatology/kex323
Close - Share Icon Share
Abstract
To describe the time interval between the onset of psoriasis and PsA in the UK primary care setting and compare with a large, well-classified secondary care cohort.
Patients with PsA and/or psoriasis were identified in the UK Clinical Practice Research Datalink (CPRD). The secondary care cohort comprised patients from the Bath PsA longitudinal observational cohort study. For incident PsA patients in the CPRD who also had a record of psoriasis, the time interval between PsA diagnosis and first psoriasis record was calculated. Comparisons were made with the time interval between diagnoses in the Bath cohort.
There were 5272 eligible PsA patients in the CPRD and 815 in the Bath cohort. In both cohorts, the majority of patients (82.3 and 61.3%, respectively) had psoriasis before their PsA diagnosis or within the same calendar year (10.5 and 23.8%), with only a minority receiving their PsA diagnosis first (7.1 and 14.8%). Excluding those who presented with arthritis before psoriasis, the median time between diagnoses was 8 years [interquartile range (IQR) 2–15] in the CPRD and 7 years (IQR 0–20) in the Bath cohort. In the CPRD, 60.1 and 75.1% received their PsA diagnosis within 10 and 15 years of their psoriasis diagnosis, respectively; this was comparable with 57.2 and 67.7% in the Bath cohort.
A similar distribution for the time interval between psoriasis and arthritis was observed in the CPRD and secondary care cohort. These data can inform screening strategies and support the validity of data from each cohort.
Rheumatology key messages
The 10 year period surrounding psoriasis onset represents a high-risk period for development of arthritis.
Trends in the time interval between psoriasis onset and PsA were similar in primary and secondary care cohorts.
Data on the time interval between psoriasis onset and PsA diagnosis can inform screening strategies.
Introduction
PsA is a chronic inflammatory arthritis affecting ∼20% of patients with psoriasis [1]. PsA is well recognized to be progressive and destructive in the majority of patients, with considerable impact on quality of life. There are now a wide range of effective treatments [2] and evidence for improved outcome with tight control in early disease [3]. Recent observational studies have provided evidence that a longer time from symptom onset to diagnosis is associated with worse radiographic and functional outcome [4, 5]. Therefore there has been increasing focus on early detection of PsA to optimize treatment and eventual outcome.
The mean age at onset of PsA is usually in the fourth decade. In the majority of patients, PsA presents after or synchronously with the onset of psoriasis [6, 7], while in a minority (13–18%) arthritis precedes psoriasis onset [6–8]. Reports to date on the incidence of PsA vary depending on the population studied and definitions used [1]. In terms of studies looking at PsA within patients with psoriasis, a recent prospective cohort study of 464 patients with pre-existing psoriasis followed over 8 years in Toronto, Ontario, Canada reported an annual PsA incidence rate of 27.0 (95% CI 21.0, 36.0) per 1000 person-years [9] while a retrospective study using medical records linkage data on 1633 psoriasis patients in Olmsted County, Minnesota, USA reported an incidence rate of 2.7 (95% CI 2.1, 3.5) per 1000 person-years [10].
An enhanced understanding of when PsA occurs in relation to psoriasis could inform targeting of population screening techniques to the highest-risk groups for inflammatory arthritis in patients with psoriasis. Our study aimed to calculate the incidence of PsA within patients with psoriasis in the UK primary care setting and describe the time interval from onset of psoriasis to PsA. Comparisons of the time interval between diagnoses in the primary care data were made with a large, well-classified secondary care cohort of patients with PsA.
Methods
Primary care cohort
The Clinical Practice Research Datalink (CPRD) contains anonymized longitudinal medical records for ∼11.7 million individuals from UK primary care [11]. It is generally representative of the UK population and has previously been used to study both psoriasis and PsA [12–14]. Incident cases of psoriasis and PsA diagnosed between 1998 and 2014 were identified where the patient was 18–89 years old at diagnosis. PsA and psoriasis cases were identified based on Read codes, which have been demonstrated to have a high positive predictive value in a similar UK database [15, 16], and prescribing data. For PsA patients, the date of diagnosis was taken as the date of the first PsA code. For patients with prescriptions for DMARDs before their first PsA code where there was no alternative indication for the prescription (such as MTX for psoriasis) (12.9%), the PsA diagnosis date was backdated to the date of the first DMARD prescription. For psoriasis patients, in addition to those with psoriasis Read codes, patients with no Read code for psoriasis but who had received two or more prescriptions for a vitamin D analogue in the absence of an alternative indication (e.g. vitiligo) were also classified as having psoriasis; these totalled 6.6% of the psoriasis cohort. The date of psoriasis diagnosis was taken as the date of the first psoriasis code or vitamin D prescription. For patients receiving psoriasis-specific treatment prior to the date of the first psoriasis code (12.5%), the diagnosis date was backdated to the date of the first psoriasis-specific prescription. Patients were classified as incident if they had ⩾1 year of research standard data before the date of diagnosis [17].
Secondary care cohort
The Royal National Hospital for Rheumatic Diseases, Bath PsA cohort was established in 1989 [18] and has recruited new and established patients fulfilling the Classification Criteria for Psoriatic Arthritis (CASPAR) from all sources, including referral from primary and secondary care. At entry into the cohort, a patient-reported background information form is completed including data on the onset of psoriasis and arthritis symptoms and diagnosis. Follow-up is based on clinical need but is generally every 3 months for those with active disease requiring treatment escalation and every 6 months for those with well-controlled disease. All patients in the cohort with patient-reported data on both the year of diagnosis of psoriasis and PsA were included in this study.
Ethical approval
Ethical approval was obtained by the CPRD data provider from a Multicentre Research Ethics Committee for all observational studies and the study protocol was approved by the CPRD Independent Scientific Advisory Committee (15_154R). For the Bath secondary care cohort, the study was approved by the Bath Research Ethics Committee and has been conducted in accordance with the Declaration of Helsinki. All participants signed informed consent. No additional approval or consent was needed.
Analysis
The age- and sex-stratified incidence rates of psoriasis and PsA in the general population were calculated (per 1000 person-years) from CPRD data. We also calculated the incidence of PsA within the incident psoriasis population who had a new diagnosis of both psoriasis and PsA between 1998 and 2014.
The time interval between each patients’ first record of psoriasis and PsA diagnosis was calculated for patients within the CPRD who had a date of diagnosis for both psoriasis and PsA. The time interval between psoriasis and PsA diagnosis was also calculated for patients in the Bath cohort and comparisons were made with the CPRD data. To take left censoring into account within the CPRD, Kaplan–Meier survival estimates were plotted from the time of first PsA diagnosis to the previous diagnosis of psoriasis in both cohorts, excluding patients where PsA and psoriasis were diagnosed synchronously and censoring CPRD data at entry into the database.
Results
Within the CPRD, 88 858 incident psoriasis (48.6% male) and 6783 incident PsA patients (49.0% male) were identified. The overall incidence of psoriasis and PsA was 1.82 (95% CI 1.81, 1.84) and 0.13 (95% CI 0.13, 0.14) per 1000 person-years, respectively. The mean duration of follow-up was 5.8 years. Within the incident psoriasis cohort the incidence of PsA was 2.73 per 1000 person-years (95% CI 2.58, 2.87). Supplementary Table S1, available at Rheumatology Online, shows the incidence rates stratified by age and sex. The mean age at PsA diagnosis was 49.4 years (s.d. 13.9).
Of the incident PsA patients in the CPRD, 5272 (77.7%) had a record of psoriasis (50.3% male). The majority of patients presented with psoriasis first (82.3%), or synchronously during the same calendar year (10.5%), with a minority presenting with arthritis first (7.1%). Excluding those who presented with PsA first, where early intervention following a psoriasis diagnosis would not be possible, the median interval between psoriasis and PsA was 8 years [interquartile range (IQR) 2–15]. Excluding also those who presented with synchronous onset or PsA first, the median interval between psoriasis and PsA was 9 years (IQR 4–17).
Complete data were available for 815 patients (90.3%) in the Bath cohort. The mean age at PsA diagnosis was 42.0 years. In total, 61.3% presented with psoriasis first, 23.8% presented synchronously within the same calendar year and 14.8% presented with PsA first. The median interval between psoriasis and PsA was 7 years (IQR 0–20). Excluding those who presented with synchronous onset, the median interval was 13 years (IQR 6–25).
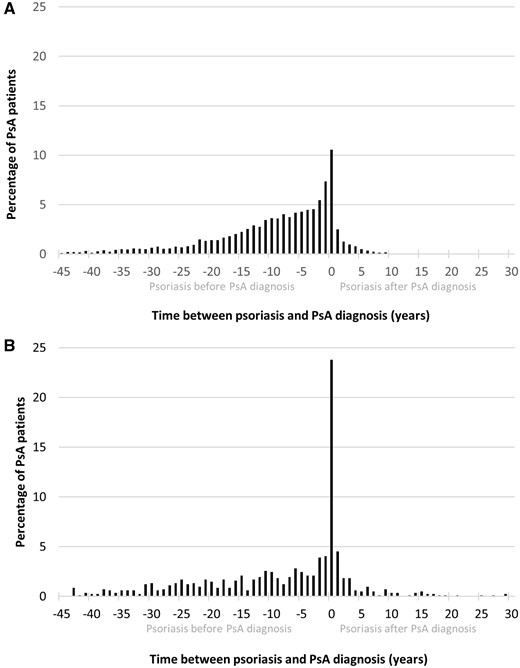
Figure 1A and B shows the interval between psoriasis and PsA diagnosis in the CPRD and Bath cohorts. In both cohorts there was a peak of synchronous onset in the same calendar year. The majority of PsA patients were diagnosed up to 15 years following their psoriasis diagnosis; including those with synchronous onset, 60.1 and 57.2% of patients were diagnosed with PsA within 10 years of psoriasis in the CPRD and Bath cohorts, respectively, and 75.1 and 67.7% were diagnosed within 15 years. Both cohorts demonstrated that the time interval between psoriasis and a later diagnosis of PsA could be >15 years, with some patients having an interval of up to 40 years.
Time interval between PsA diagnosis and the first record of psoriasis
(A) in the CPRD and (B) in the Bath secondary care cohort.
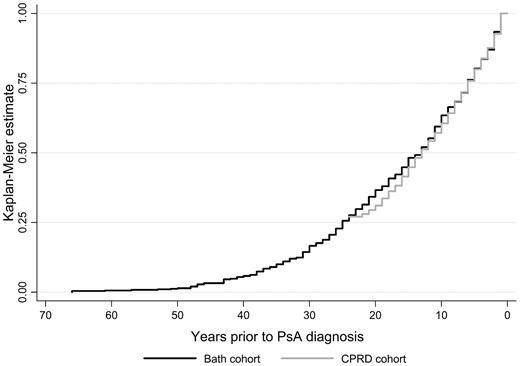
Figure 2 shows the Kaplan–Meier survival curves for the CPRD and the Bath cohorts taking into account left censoring in the CPRD cohort and excluding those with synchronous onset. There was no significant difference in the survival estimates for the two cohorts (log-rank test for difference, P = 0.26). The median time interval between psoriasis and PsA diagnosis was 13 years for both cohorts (95% CI 12, 15 for the Bath cohort and 95% CI 12, 14 for the CPRD cohort).
Time from PsA diagnosis to previous psoriasis diagnosis using Kaplan–Meier estimates for the Bath and CPRD cohorts (log-rank test for difference, P = 0.26). All included patients had a diagnosis of both psoriasis and PsA. In the CPRD cohort there was a maximum of 24 years follow-up prior to a PsA diagnosis before a patient’s left censor date, while in the Bath cohort there was no left censoring and some patient-reported time intervals were >60 years.
Discussion
Similar trends in the time interval between psoriasis onset and PsA diagnosis were seen in the primary and secondary care cohorts, with the majority of cases of PsA occurring synchronously with or within 10 years of the onset of psoriasis. The greater proportion of synchronous onset within the same calendar year seen in the Bath cohort (23.8% compared with 10.5% in the CPRD) may relate to the detection of mild psoriasis (unnoticed or not of concern to the patient) by a clinician examining for arthritis in secondary care. Irrespective of the reason, these findings demonstrate that the 10 year period of time surrounding the onset of psoriasis represents a high-risk period for the development of arthritis and as such is potentially the best time for enhanced surveillance. Similarly, it is helpful on a clinical level to be aware of when the highest-risk times are for developing PsA as well as recognizing that the disease can present many years after psoriasis.
The findings that the majority of PsA patients are diagnosed following psoriasis and that some patients develop PsA many years after their psoriasis diagnosis are in line with other studies. The incidence of PsA within the incident psoriasis cohort in the CPRD was in line with a study by Love et al. [19] using data from a similar UK primary care database and a study using data from the Rochester Epidemiology Project medical records linkage system [10]. However, the incidence was 10 times lower than that reported in the prospective Toronto psoriasis cohort study [9]. This difference may be explained in part by the incident nature of the CPRD psoriasis cohort, combined with the length of the study period, resulting in patients having a shorter psoriasis disease duration than those in the Toronto study. The Toronto study also recruited >65% of patients from phototherapy centres, likely capturing those with more severe psoriasis, which has been found to be associated with an increased risk of PsA [9, 20]. In addition, all patients in the Toronto cohort underwent an annual review by a rheumatologist, allowing them to diagnose early and milder PsA cases before a patient may have presented to a doctor. Nevertheless, while the CPRD population may be more representative of the psoriasis population as a whole, the large discrepancy may suggest underascertainment of PsA within the CPRD and standard health care setting and provide support for previous studies reporting a high burden of undiagnosed PsA within patients with psoriasis [8].
The similarities between the CPRD and secondary care cohort provide support for the validity of data from each cohort that capture data from different health care settings. Within the CPRD, the date of psoriasis diagnosis was taken as the first record of psoriasis or a prior prescription for a psoriasis-specific treatment. The nature of data capture and censoring within the CPRD and the variability of psoriasis in terms of both activity and severity mean that it is possible that the record taken as the psoriasis diagnosis date may not actually relate to the time of disease onset. Mild disease may go unreported to the general practitioner (GP) for some time or a GP may enter a first record of psoriasis when a patient joins the practice but not backdate it to the time of first symptom onset. However, the similar distribution of time to PsA diagnosis within the two datasets provides support for the coding of psoriasis and PsA in the CPRD and the method of backdating the diagnosis date based on the issue of an earlier prescription for a product used to treat psoriasis or PsA. Similarly, the retrospective nature of data collection on the timing of psoriasis and PsA diagnoses within the Bath cohort may make it subject to recall bias; however, these data support the view that the impact of any recall bias is likely to be minimal.
Conclusion
We report similar distributions for the interval between psoriasis and PsA in the CPRD and a well-classified secondary care cohort. Although the majority in both cohorts receive a diagnosis of PsA within 10 years of the diagnosis of psoriasis, there is a significant proportion who do not. These data can inform screening strategies and support the validity of data from each cohort.
Acknowledgements
We acknowledge contributions from Jana James (PsA research partner), Nicola Waldron (PsA nurse specialist), Charlotte Cavill (database manager) and Mandy Knight (database administrator). We wish to acknowledge the non-contributing authors of the PROMPT (early detection to imPRove OutcoMe in people with undiagnosed Psoriatic arthriTis) study group who have been responsible for the acquisition of funding and general supervision of the research group: Sarah Hewlett, Helen Harris, Philip Helliwell, Laura Coates, Catherine Fernandez, Sarah Brown, Claire Davies, Jonathan Packham, Laura Bjoke, Eldon Spakman, Anne Barton, Oliver Fitzgerald, Vishnu Madhok, Melanie Brooke, Jana James and Andrew Parkinson.
Funding: This report is independent research funded by the National Institute for Health Research [Programme Grants for Applied Research, Early detection to improve outcome in patients with undiagnosed psoriatic arthritis (grant number RP-PG-1212-20007)]. The views expressed in this publication are those of the authors and not necessarily those of the National Health Service, the National Institute for Health Research or the Department of Health.
Disclosure statement: A.N. Reports grants from Pfizer and Celgene outside the submitted work. C.S. has received departmental funding from manufacturers of drugs used to treat psoriasis and psoriatic arthritis including AbbVie, Pfizer, Novartis, Roche, Regeneron and Janssen. All other authors have declared no conflicts of interest.
Supplementary data
Supplementary data are available at Rheumatology Online.
References
Author notes
on behalf of the PROMPT study group
William Tillett and Rachel Charlton contributed equally to this study.



Comments