Article Text
Abstract
Objective To investigate whether temporary discontinuation of methotrexate (MTX) improves the efficacy of seasonal influenza vaccination in patients with rheumatoid arthritis (RA).
Methods In this prospective randomised parallel-group trial, patients with RA taking stable dose of MTX were randomly assigned at a ratio of 1:1:1:1 to continue MTX (group 1), suspend MTX for 4 weeks before vaccination (group 2), suspend MTX for 2 weeks before and 2 weeks after vaccination (group 3) or suspend MTX for 4 weeks after vaccination (group 4). All participants were vaccinated with trivalent influenza vaccine containing H1N1, H3N2 and B-Yamagata. The primary outcome was frequency of satisfactory vaccine response (≥4-fold titre increase 4 weeks postvaccination). Secondary endpoints included fold change in antibody titres from baseline.
Results The per-protocol population consisted of 199 patients (n=54, 44, 49 and 52 in groups 1, 2, 3 and 4, respectively). Group 3 achieved higher satisfactory vaccine response against all three antigens than group 1 (51.0% vs 31.5%, p=0.044). The anti-H3N2 antibody fold increase (95% CI) was significantly higher in groups 3 and 4 (12.2 (8.4 to 17.5), p <0.001 and 10.0 (6.8 to 14.8), p=0.043, respectively) than group 1 (5.9 (4.3 to 8.1)). The anti-B-Yamagata antibody responses of groups 3 and 4 were higher (4.7 (3.3 to 6.7), p=0.048; 6.1 (4.2 to 8.8), p <0.001, respectively) than group 1 (2.9 (2.2 to 3.8)). RA flare occurred in 24.1%, 21.2%, 34.1% and 38.8% in groups 1, 2, 3 and 4, respectively (p=NS).
Conclusions Temporary MTX discontinuation improves the immunogenicity of seasonal influenza vaccination in patients with RA.
Trial registration Trial registration number is: www.clinicaltrials.gov, NCT02748785.
- rheumatoid arthritis
- methotrexate
- vaccination
- influenza.
Statistics from Altmetric.com
Introduction
Rheumatoid arthritis (RA) is a chronic systemic inflammatory disease in which the main target of inflammation is the joints. Patients with RA require chronic treatment with disease-modifying antirheumatic drugs (DMARDs), which constitute the mainstay of treatment. Patients with RA are more susceptible to infections because of their underlying immune dysfunction and the treatment-induced immune suppression.1 2 Consequently, they are recommended to receive vaccines against preventable diseases, including influenza.3 4
Methotrexate (MTX) is highly effective and is recommended in almost all patients with RA.5 6 However, MTX significantly decreases the satisfactory response of patients with RA to pneumococcal and seasonal influenza vaccination.7–10 Moreover, it has a particularly negative effect on the response to presumably novel antigens such as new pandemic H1N1 influenza antigen.10 Consequently, patients with RA are recommended to be vaccinated before they are treated with MTX.11 However, most patients with RA are already receiving MTX at the time when vaccination is required. Therefore, a novel strategy to improve the vaccine response of such patients with RA is needed.
The present clinical trial aimed to investigate whether discontinuing MTX for 4 weeks before, during or after seasonal influenza vaccination improves the immunogenicity of the vaccine in patients with RA who are being treated with a stable dose of MTX.
Methods
Study
This was a prospective single-centre randomised single-blind parallel-group intervention study that aimed to investigate the effects of temporary MTX discontinuation on vaccine response to seasonal influenza vaccination in patients with RA. The study started in September 2015 and was completed in July 2016.
After obtaining informed consent, the patients were screened for eligibility according to the inclusion and exclusion criteria described below. The study was approved by the institutional review board of the Seoul National University Hospital (IRB 1508-050-694) and was conducted in accordance with the principles of the Declaration of Helsinki and Good Clinical Practice guidelines.12 The study was registered at www.clinicaltrials.gov (protocol number NCT02748785).
Patients
Patients with RA who were aged 18 years or older and had been on the same dose of MTX for 6 weeks or longer were eligible for inclusion. RA was defined on the basis of revised 1987 American College of Rheumatology criteria.13 The exclusion criteria were as follows: pregnant or lactating women, patients with a previous anaphylactic response to vaccine components or to egg, evidence of an acute infection with temperature >38°C at the time of vaccination, history of Guillain-Barré syndrome or demyelinating syndromes, and previous vaccination with any live vaccine 4 weeks before or any inactivated vaccine 2 weeks before start of the study. Patients with high disease activity that necessitated a recent change in their treatment regimen and patients with any other additional rheumatic disease except for secondary Sjogren’s disease were also excluded.
Randomisation/blinding
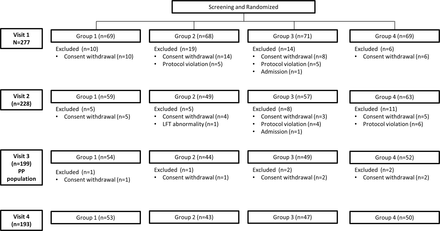
The eligible patients were randomly assigned to continue MTX (group 1), suspend MTX for 4 weeks before vaccination (group 2), suspend MTX for 2 weeks before and 2 weeks after vaccination (group 3), or suspend MTX for 4 weeks after vaccination (group 4) (figure 1). Patients were assigned to these treatment groups by a Central Interactive Web Response System at a 1:1:1:1 ratio according to the randomisation table. Information on the intervention was concealed from the investigators who enrolled and assessed the study patients. To measure the adherence to the study protocol, study participants were required to record their MTX administration in a diary.
Study design. MTX, methotrexate.
Intervention
The seasonal trivalent influenza vaccine (GC Flu, Green Cross, South Korea) contained 15 µg of A/California/72009 Reassortant virus NYMC X-181 (H1N1), 15 µg of A/Switzerland/9715293/2013 Reassortant virus NIB-88 (H3N2) and 15 µg of B/Phuket/3073/2013 (B-Yamagata). The vaccine was contained in a 0.5 mL prefilled syringe and was delivered as a single intramuscular injection in the deltoid muscle.
Baseline, treatment and follow-up visits
There were four visits. Visit 1 (week 0) took place 4 weeks before vaccination. During visit 2 (week 4), the prevaccination sera were taken and all patients were vaccinated. Visit 3 (week 8) took place 4 weeks after vaccination, at which point the postvaccine antibody titres were measured. There was also a fourth visit 16 weeks after vaccination to assess disease activity (week 20) (figure 1).
Concomitant medications
Adding or changing DMARDs were not allowed until the postvaccination antibody titre was obtained (ie, 4 weeks after the vaccination, visit 3). Medications for other comorbid conditions were continued. During MTX discontinuation, acetaminophen (650 mg up to three times per day)/nonsteroidal anti-inflammatory drugs (NSAIDs) (in standard dosing) and prednisolone (or its equivalent) up to 10 mg per day were allowed for RA flares.
Efficacy and safety assessments
The primary outcome was the frequency of satisfactory vaccine response to influenza antigens 4 weeks after vaccination (ie, visit 3). A satisfactory vaccine response was defined as a ≥4-fold increase in haemaglutination inhibition (HI) antibody titre at visit 3 relative to the prevaccination HI antibody titre at visit 2. Secondary endpoints were (1) fold change in postvaccination HI antibody titres against each vaccine antigen at visit 3 relative to baseline, and (2) frequency of patients who lacked seroprotection at baseline (defined as HI titres of <1:40) who became seroprotected against each vaccine antigen at visit 3 (defined as HI titres of ≥1:40).14
The 28-joint disease activity score (DAS28) was measured and adverse events that were associated with vaccination were captured from the patients at each visit. An RA flare was defined as an increase in DAS28 of >1.2 (or >0.6 if the baseline DAS28 was ≥3.2).15
Titre measurements
The HI antibody titres against each of the three influenza strains in the vaccine were measured in duplicate by an independent laboratory (GC Labs, Gyeonggi-do, South Korea) according to standard procedures. The average of the duplicate measurements for each antigen was calculated.
Statistical analysis
Analysis and safety population
The primary analysis population (per-protocol population) included all study subjects who underwent the vaccination, discontinued or continued MTX according to the allocated regimen, and whose prevaccination and postvaccination titres were available.
Sample size calculation
The vaccine response to influenza, defined as a fourfold or more increase in HI antibody titres in two or more of three influenza antigens, in patients with RA with and without concurrent MTX treatment has been reported to be 61.8% and 76.7%, respectively.7 Assuming that 4 weeks of MTX discontinuation would improve the vaccination response to that seen in patients without MTX treatment, and assuming an alpha level of 0.05 (two-tailed), a power of 0.90 and dropout rate of 10%, 146 patients per group would be required for the study. Thus, the total target number was 584 patients.
Continuous variables were analysed by using a t test or Mann-Whitney U test, as appropriate. The binary secondary efficacy variables (frequency of satisfactory vaccine response and frequency of disease flare) were analysed by using χ2 tests or Fisher’s exact test, as appropriate. p<0.05 was considered to indicate statistical significance. Analyses were not adjusted for multiple testing because these analyses were performed with exploratory rather than confirmatory intention. All analyses were performed by using SPSS (IBM SPSS Statistics V. 18).
Results
Baseline characteristics of the patients
Patients were asked to participate during their longitudinal follow-up in the Rheumatology Clinic between September 2015 and November 2015. A total of 277 patients were randomly assigned to the four treatment groups. The target participant number (n=584) could not be reached due to the short enrolment period (3 months): it was short because the patients had to be vaccinated before the start of the influenza season (ie, by the end of December 2015). The per-protocol population consisted of the 199 patients who had at least completed visit 3 (figure 2). The patients were predominately female. The four groups did not differ at baseline in terms of demographic or disease characteristics, including DAS28 C reactive protein. The groups were also comparable in terms of their treatment regimen at baseline, including their use of oral corticosteroids and MTX (table 1).
Patient flow.
Baseline characteristics of the study patients (the per-protocol population)
Effect of MTX discontinuation on influenza vaccine efficacy
The four groups were similar in terms of their baseline HI antibody titres against H1N1, H3N2 and B antigens. Four weeks after the vaccination (visit 3), the patients in all four groups mounted significant humoral immune responses against the three vaccine antigens (see online supplementary table S1).
Satisfactory vaccine response
The four groups were similar in terms of the frequency with which they mounted a satisfactory vaccine response (defined as an increase relative to baseline of at least fourfold) to at least one influenza antigen (figure 3A). However, group 3, in which MTX treatment was suspended 2 weeks before and 2 weeks after the vaccination, tended to respond more satisfactorily to at least two influenza antigens than group 1 (no MTX suspension) (figure 3B). Group 3 also responded significantly more satisfactorily to all three antigens than group 1 (figure 3C). Groups 2 (MTX suspension for 4 weeks before vaccination) did not differ significantly from group 1 in terms of these responses, whereas group 4 (MTX suspension for 4 weeks after the vaccination) tended to respond better than group 1 (figure 3).
Frequency of satisfactory vaccination responses to the three influenza antigens in the vaccine. Satisfactory vaccine response was defined as a ≥4-fold improvement in titres relative to baseline. The numbers in the bars indicate the percentage of satisfactory responders. p Values were generated by Mann-Whitney U tests.
Vaccine response to individual strain
In terms of the responses to individual vaccine antigens, groups 3 and 4 responded satisfactorily to B-Yamagata more frequently than group 1 (group 3 vs group 1: difference, 20.3%; 95% CI, 1.0% to 39.6%; p=0.040; group 4 vs group 1: difference, 22.6%; 95% CI, 3.6% to 41.7%; p=0.0197). Differences between the groups in responses to H1N1 or H3N2 were not observed (see online supplementary table S1 and supplementary figure S1A). Analysis of the subgroup of patients whose baseline HI antibody titre was <1:40 showed that group 3 responded (ie, HI antibody titre ≥1:40) to all three antigens significantly more frequently than group 1 (see online supplementary table S2, supplementary figure S1B).
Fold change in antibody titres
Compared with group 1, groups 3 and 4 had significantly higher fold increases in their antibody titres against H3N2 and B-Yamagata antigen, but not against H1N1 antigen (figure 4A). The anti-H3N2 antibody responses of groups 3 and 4, but not group 2, improved better relative to baseline (12.2-fold, 95% CI=8.4 to 17.5, p<0.001; 10.0-fold, 95% CI=6.8 to 14.8, p=0.043; and 6.1-fold, 95% CI=4.4 to 8.5, p=0.859, respectively) than group 1 (5.9-fold, 95% CI=4.3 to 8.1). Similarly, the anti-B-Yamagata antibody responses of groups 3 and 4, but not group 2, improved better (4.7-fold, 95% CI=3.3 to 6.7, p=0.048; 6.1-fold, 95% CI=4.2 to 8.8, p<0.001; and 2.8-fold, 95% CI=2.1 to 3.7, p=0.795, respectively) than group 1 (2.9-fold, 95% CI=2.2 to 3.8). Groups 3 and 4 also exhibited numerically greater fold changes in anti-H1N1 antibody titre (8.7-fold, 95% CI=5.3 to 14.5; 8.1-fold, 95% CI=5.3 to 14.4, respectively) than groups 1 and 2 (5.1-fold, 95% CI=3.4 to 7.8; 5.0-fold, 95% CI=3.2 to 7.8), although these differences did not achieve statistical significance.
Fold change in antibody titres relative to baseline (box-and-whisker plots) and seroprotection rates before and after vaccination (frequencies below the plot) in all patients (A) or only patients whose baseline antibody titre was <1:40 (B). Satisfactory vaccine response was defined as a ≥4-fold improvement in titres relative to baseline and is indicated by the dotted horizontal line in the box-and-whisker plots. The boxes represent the IQR. The median is represented by the horizontal line. The whiskers represent the 10th and 90th percentiles. The groups were compared in terms of fold change by using the Mann-Whitney U test. Seroprotection was defined as titres of ≥1:40. n, number; post-SP, postvaccination seroprotection rate; pre-SP, prevaccination seroprotection rate.
The differences between groups became more prominent when the analysis was restricted to patients who lacked seroprotection before vaccination (ie, HI antibody titres of <1:40): group 3 had significantly higher fold increases in antibody titres against all three antigens than group 1. Group 4 also exhibited significantly higher fold increases in antibody titres against B-Yamagata than group 1 (figure 4B). Differences between groups were not observed when only the patients with seroprotection at baseline were analysed (see online supplementary table S3). Group 2 did not differ from group 1, regardless of the vaccine efficacy variable.
Seroprotection
Notably, the overall baseline seroprotection against H1N1 and H3N2 appeared to be higher than that against B-Yamagata (table 2). In terms of seroprotection after vaccination, >95% of the patients became seroprotected against H3N2 regardless of group (figure 4 and see online supplementary table S1). However, groups 3 and 4 became seroprotected against H1N1 and B-Yamagata more frequently than group 1. The difference was more prominent in the patients who lacked seroprotection at baseline (figure 4B, see online supplementary table S2).
Adverse events
Safety
The vaccine was well tolerated. No severe adverse events that related to the vaccination were reported during follow-up. Of the 199 patients, 58 (29.1%) experienced a RA disease flare during the study. Flares tended to be more common in groups 2 (n=15; 34.1%) and 3 (n=19; 38.8%), while groups 1 and 4 had lower flare rates (n=13; 24.1% and n=11; 21.2%, respectively). However, these differences did not achieve statistical significance (p=0.224). Most patients recovered from the flare except one who exhibited a higher disease activity than at baseline at the end of the study (table 2).
Discussion
In this study, we showed that temporarily discontinuing MTX, especially when the vaccination occurred in the middle of the discontinuation period, significantly increased the efficacy of a seasonal influenza virus vaccine in patients with RA who were on a stable dose of MTX. To the best of our knowledge, this study is the first to demonstrate a novel strategy that increases the vaccine immunogenicity in such patients with RA.
The immunosuppressive effects of MTX might explain why this drug associates with impaired vaccine responses and, more beneficially, why it prevents the development of antidrug antibodies.16–18 Notably, the therapeutic efficacy of MTX in RA takes weeks to months to achieve maximum efficacy and is sustained for several weeks after discontinuation. This suggests that MTX has a considerably long biological half-life.19 While this suggests that short-term discontinuation of MTX would not boost the response of patients with RA to vaccines, we found that temporary MTX discontinuation (4 weeks) did improve the vaccine response of patients who had been on a stable dose of MTX. Furthermore, we showed that the time point of MTX discontinuation was critical: compared with patients without MTX discontinuation, the response to vaccine was greatest when MTX was suspended for 2 weeks before and 2 weeks after vaccination (group 3). By contrast, and somewhat counterintuitively, discontinuing MTX for 4 weeks before the vaccination (group 2) did not improve it. Moreover, discontinuing MTX for 4 weeks after the vaccination (group 4) was quite effective, although less effective than group 3. These data suggest that the effect of MTX on immune cells is actually immediate, whereas the disease-modifying effects of MTX (ie, the inhibition of inflammation in the joints) may take significantly longer to evolve.20–22 The fact that discontinuing MTX improves immune responses to vaccines also supports the clinical practice that MTX be discontinued during acute (life-threatening) infections.
Notably, the overall baseline immunogenicity against H1N1 and H3N2 appeared to be higher than that against B-Yamagata (table 2). This suggests that patients had not been previously uniformly exposed to the three influenza antigens. Alternatively, B-Yamagata antigen is less immunogenic. In terms of seroprotection after vaccination, >95% of the patients were seroprotected against H3N2 regardless of group (table 2). In terms of H1N1 and B-Yamagata, groups 3 and 4 became seroprotected against more frequently than group 1. The difference was more prominent in the patients who lacked seroprotection at baseline.
In all groups, the vaccine response to the three influenza antigens varied markedly: there were strong responses to H3N2, lower responses to H1N1 and the weakest response was to B-Yamagata. The response to the less immunogenic B-Yamagata exhibited the best improvement (figure 4), indicating that vaccine response to less immunogenic antigens is suppressed by MTX. In addition, MTX discontinuation particularly improved the response to vaccine when the patients lacked protective antibody titres before vaccination (figure 4B). This suggests that the MTX discontinuation may have an even greater impact on the immune response to new, less immunogenic, antigens or vaccines that immune system has not encountered before or has not responded to appropriately previously. This possibility has important clinical implications. MTX discontinuation will improve responses to vaccines that are based on a new influenza pandemic strain and it will also help infected patients to generate immune responses to such strains more rapidly.23 MTX discontinuation might aid vaccination against antigens with low immunogenicity, such as herpes zoster.24 Further studies that assess the efficacy and safety of temporary MTX discontinuation in various clinical settings are warranted.
The majority of patients tolerated the influenza vaccination well without major complications. However, MTX discontinuation did associate with more RA flares compared with MTX continuation. However, nearly all of the patients recovered fully from their flare after MTX was reintroduced (table 2). Further studies that determine the optimal duration of MTX discontinuation that improves vaccine efficacy while avoiding RA flares are warranted.
The study has several limitations. First, since it was a single-centre study, it was not possible to enrol the optimal number of patients needed to identify the best MTX discontinuation regimen. The number of enrolled patients was fewer than the initial target sample size of 584, resulting in a power of 0.46 to detect a difference in vaccine response to at least two antigens and 0.52 for response to all three antigens. Second, we only enrolled patients with stable RA and low disease activity on a relatively low MTX dose. Moreover, all patients had the Korean ethnicity. Further studies testing the generalisability of our results to patients with moderate–high disease activity or with other ethnicities are needed. Last but not least, it remains unclear as yet whether a rise in antibody titre actually translates into decreased influenza incidence, although the HI antibody titres have been shown as a correlate of vaccine-induced protection.25
In conclusion, temporary discontinuation of MTX was associated with improved humoral vaccine response to a seasonal influenza vaccine in patients with RA who were receiving a stable dose of MTX. Further studies are needed to determine whether MTX discontinuation decreases the influenza incidence or alter the course of the disease.
References
Footnotes
Contributors EBL had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. EBL and JKP: study concept and design. JKP, MAL, EYL, YC, YWS, KLW and EBL: acquisition, analysis or interpretation of data. EBL, KLW and JKP: drafting of the manuscript. JKP, KHL, EYL, YWS, YC, KW and EBL: critical revision of the manuscript for important intellectual content. EBL, JKP, YC and KLW: statistical analysis. EBL: obtained funding, study supervision.
Funding This research was partly supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2016R1D1A1A02937044).
Competing interests EBL has acted as a consultant to Pfizer. The other authors declare no conflicts of interest.
Patient consent Obtained.
Ethics approval The study was approved by the institutional review board of the Seoul National University Hospital [IRB 1508-050-694] and was conducted in accordance with the principles of the Declaration of Helsinki and Good Clinical Practice guidelines. All patients provided written informed consent.
Provenance and peer review Not commissioned; externally peer reviewed.