Article Text
Abstract
Objective The aim of this study was to define characteristic MRI findings in the spine of patients with axial spondyloarthritis (SpA) and provide a definition of a positive spinal MRI for inflammation and structural changes.
Methods Technical details of spinal MRI and the description of spinal lesions of both inflammation and structural changes were discussed in consecutive meetings of 10 experts of the Assessment in SpondyloArthritis international Society (ASAS). The discussions aimed at a broad consensus on definitions of ‘a positive spinal MRI’ for both types of lesions and were backed up by a systematic literature search.
Results A total of six different types of lesions were described for inflammation—anterior/posterior spondylitis, spondylodiscitis, arthritis of costovertebral joints, arthritis of zygoapophyseal joints and enthesitis of spinal ligaments—and another four for structural changes—fatty deposition, erosions, syndesmophytes and ankylosis. In the literature review, four relevant papers were identified. Anterior/posterior spondylitis and fat depositions at vertebral edges were considered as the most typical findings in SpA. Based on expert consensus and taking the literature review into consideration, a positive spinal MRI for inflammation was defined as the presence of anterior/posterior spondylitis in ≥3 sites. Evidence of fatty deposition at several vertebral corners was found to be suggestive of axial SpA, especially in younger adults. ASAS members (n=56) approved these definitions by voting in January 2010.
Conclusions This consensus statement gives clear descriptions of disease-related spinal lesions and of definitions of a positive spinal MRI for inflammatory lesions (spondylitis) and structural changes (fat deposition). These definitions can be used to describe findings of spinal MRI in patients with SpA in daily practice and clinical studies.
Statistics from Altmetric.com
Introduction
Imaging of the spine plays an important role in the diagnosis, classification and monitoring of ankylosing spondylitis (AS) and other spondyloarthritides (SpAs).1 Since the 1990s, MRI has been increasingly used to visualise inflammation in the sacroiliac (SI) joints and in the spine of patients with AS.2 Inflammation seen on MRI of SI joints and spine seems to be related to structural changes seen on plain radiographs,3 ,4 but the exact sequence of events is as yet incompletely understood.
Definite structural changes in the SI joints but not of the spine are part of current classification criteria for AS.5 ,6 However, since spondylitis may occur in the absence of definite structural changes in the SI joints,7 a clinical diagnosis of AS in an individual patient with characteristic clinical symptoms is possible if syndesmophytes are present.
The new classification criteria for axial SpA include SI inflammation on MRI as a major criterion for the classification of patients with chronic low back pain and age at onset ≤45 years in the imaging part of this criteria set.8 ,9 Subsequently, a definition of ‘a positive MRI for the SI joints’ was developed for patients with SpA by the Assessment in SpondyloArthritis international Society–Outcome Measures in Rheumatology (ASAS/OMERACT) MRI working group.10 Since there is evidence that spondylitis may also occur prior to—or even without—sacroiliitis,9 ,11 a definition of a ‘positive MRI’ for spinal inflammation was also considered necessary. In order to homogenise the classification of patients with SpA throughout the world, we felt the need to internationally agree on definitions of both inflammatory and structural changes in the spine of patients with axial SpA including AS.
Therefore, the ASAS/OMERACT working group has initiated this process to reach consensus within this group of experienced rheumatologists and radiologists. In detail, the aims were fivefold: (1) to agree on minimal technical requirements, (2) to define which MRI findings can be considered characteristic of SpA and AS, (3) to provide representative MR images, (4) to agree on criteria for a positive inflammatory signal in the spine and (5) to point out and discuss differential diagnoses.
Methods
Members of the ASAS/OMERACT working group who recently assessed MRI scoring instruments for the SI joints and spine,12 ,13 as well as the definition of a positive MRI for the SI joints,10 were invited to participate in this consensus approach. The final group consisted of 10 persons, 2 radiologists and 8 rheumatologists, all with clinical and scientific experience in both MRI and SpA. This group held a total of four workshops in Berlin between February 2008 and January 2010. Official endorsement by the members of the ASAS was sought during the annual workshop, which took place 15/16 January 2010 in Berlin. The preparations for the meetings and the coordination were done by KGH and JB.
In general, the process of the group for a consensus statement of the SI joints was applied and modified for the spine. Briefly, during the workshop meetings, MR images depicting numerous pathological findings as well as normal variants of the spine were discussed, representative MR images were selected for illustration and a definition of ‘cut-off values’ searched for findings that should allow a dichotomous decision of whether a certain MRI is ‘positive’ or not in terms of findings attributable to SpA.10
In addition to the discussions during the workshops and via email, a systematic literature review was performed by KGH to retrieve relevant papers on SpA and AS dealing with MRI of the spine. Details of the literature search are outlined in figure 1. Briefly, recent papers (published in the year 2000 or later, and before 30 June 2011) dealing with diagnosis of SpA by MRI with proper study design and using a non-SpA control group were considered for detailed analysis. In a first step, Medline was searched and search criteria were formulated to include only original reports (see supplemental online material for full search string). This resulted in 410 papers. All abstracts of these papers were downloaded and the selection of papers was manually narrowed down based on criteria outlined in figure 1.
Diagrammatic presentation of structured literature search. *Medline search string available in supplemental material of this article.
Results
The group first decided to differentiate inflammatory lesions from structural lesions. Of note, the term ‘active’ inflammation was avoided to make a clear distinction to structural changes that are lesions regarded as a sequel to or result of inflammation.
Thereafter, agreement on the following aims was achieved:
definition of the minimal technical requirements for assessment of inflammatory and structural spinal lesions in AS
description of the different spinal inflammatory lesions typically involved in AS
agreement on the definition of ‘positive MRI’ for inflammatory lesions of the spine in AS/axial SpA
agreement on definitions of structural changes in spinal MRI of patients with SpA
description of important differential diagnoses.
There was a general consensus that it is possible and necessary to differentiate inflammatory from structural spinal changes. To date, MRI is the only imaging technique that can reliably detect inflammation in the spine. In contrast, structural changes are also well detected by radiography and CT, as well as by MRI.14 Different MRI techniques are needed to depict and differentiate active lesions and structural changes. To detect inflammation, we used MRI techniques that are able to visualise the increased water content of a tissue, hypercellularity and/or hypervascularity. The detection of structural changes by MRI is, in general, possible but hampered by the fact that MRI techniques are mainly based on the depiction of fat whereas calcification and cortical bone growth cannot be directly visualised.
Technical aspects
Using MRI, T1-weighted (T1w) sequences are usually applied to evaluate structural changes. For the detection of inflammatory changes, a variety of techniques can be used: (1) short τ inversion recovery (STIR) sequence, (2) T2-weighted (T2w) fat-suppressed fast spin echo (FSE) sequence (T2w/FS) and (3) T1w fat-suppressed FSE after administration of paramagnetic contrast medium like gadolinium diethylenetriamine pentaacetic acid (Gd-DTPA) (T1w/Gd). Using these techniques, inflammatory spinal lesions are depicted as hyperintense/bright areas, whereas normal bone marrow appears dark.15 The majority of lesions are equally well depicted by all three MRI sequences listed above.16 ,17
However, there are different underlying technical principles. Both T2w/FS and STIR sequences depict areas of increased water content as hyperintense signal, for example, bone marrow oedema. With regard to the information obtained with the two latter sequences, there are subtle differences: the STIR sequence has a lower signal-to-noise ratio as compared to T2w/FS—which results in a slightly lower image quality—but the advantage is that this sequence is less prone to artefacts. In the following, lesions are described by their appearance in STIR sequences only. However, the other two types of sequences for depicting inflammatory changes may also be used.
When using T1w/Gd sequences, inflammatory lesions are depicted bright on the basis of diffusion of Gd-DTPA molecules into the interstitium, which is due to increased vascularity associated with inflammation. The need for injecting contrast media constitutes the major disadvantage of this technique, which is more invasive, more time consuming and more costly than the other two. Furthermore, the application of contrast agents is contraindicated in patients with severe renal insufficiency.18 On the other hand, additional diagnostic information may be obtained using T1w/Gd in selected patients.19
MRI machines with 1.0–3.0 Tesla field strength may be used for imaging of the spine. The majority of studies have been performed using 1.5-Tesla machines. A sample sequence protocol defining minimal specifications is given in table 1.
Sample sequences for 1.5-Tesla MR scanners
A slice thickness of maximally 4 mm is recommended in all MRI sequences.
The standard orientation for the spine is the sagittal plane, and slices should cover the entire vertebral body from one side to the other including the posterior elements (zygoapophyseal, costovertebral joints and spinous processes).
Additional sequences in transverse or coronal planes may provide additional information but are not needed in daily routine for screening purposes.
Spinal locations
Inflammatory lesions
The vast majority of active MRI lesions in the spine in SpA are seen in the bone usually related to entheseal structures at the intervertebral disc. These lesions are designated as osteitis/bone marrow oedema. Enthesitis occurs at sites where ligaments and tendons attach to bone and is usually associated with osteitis. Currently, it is unclear whether synovitis of the zygoapophyseal, costovertebral and costotransverse joints on MRI as a single feature without concomitant osteitis does occur. Thus, the most important concepts of SpA are osteitis/bone marrow oedema and enthesitis.
The structures that may be involved in AS are as follows:
vertebral bodies
intervertebral discs
zygoapophyseal joints (facet joints)
costovertebral and costotransverse joints
insertion sites of spinal ligaments (supraspinal ligament, interspinal ligament, ligamenta flava).
In clinical practice, inflammation of the above-mentioned anatomical structures is referred to as
spondylitis
spondylodiscitis
arthritis of the zygoapophyseal (facet) joints
arthritis of the costovertebral and costotransverse joints
enthesitis of spinal ligaments.
Definitions of these different inflammatory lesions are given in table 2.
Locations and descriptions of inflammatory changes
Anterior spondylitis. (A) STIR image showing corner-related osteitis (white arrows) at T12 to L3. (B) The corresponding T1 image shows signal loss in the affected areas. In addition, there is fatty marrow infiltration at the upper endplate of T12 (white arrowhead). STIR, short τ inversion recovery.
Posterior spondylitis. (A) STIR image showing triangular-shaped, corner-related osteitis (white arrows). (B) Corresponding T1 image shows signal loss of the affected areas. STIR, short τ inversion recovery.
Spondylodiscitis. (A) STIR image with hemicircular osteitis at lower endplate of L2 (white arrow). (B) Corresponding T1 image shows signal loss (white arrow). STIR, short τ inversion recovery.
Arthritis of costovertebral joints. STIR image showing osteitis in the posterior aspects of T5–9 and within the pedicles due to arthritis of costovertebral joints (white arrows). In addition, posterior spondylitis is seen in T11 and anterior spondylitis in L3 (white arrowheads). STIR, short τ inversion recovery.
Arthritis of zygoapophyseal joints. (A) STIR image showing osteitis within the pedicles of T12, L1 and L3 (white arrows) as a sign of inflamed facet joints. (B) Corresponding T1 image showing only very minute signal loss of affected pedicles. STIR, short τ inversion recovery.
Enthesitis. Fat-suppressed T1w sequence after contrast medium injection showing soft tissue inflammation at attachment site of supraspinal ligament at spinous process of C7 (white arrowheads) and accompanying osteitis (white arrow). In addition, zygoapophyseal joint arthritides are visible at C1 through C5 (black arrowheads). T1w, T1-weighted.
Each of the inflammatory lesions described must be visible in at least two or more consecutive sagittal slices.
Inflammatory lesions: summary
As a result of consensus discussions and after considering the published evidence,11 ,20 the group agreed on the following statements:
Anterior and posterior spondylitis (corner-based inflammatory lesions) are typical for axial SpA.
Evidence of anterior/posterior spondylitis in three or more sites is highly suggestive of axial SpA, especially in the younger age group (where degenerative changes play a minor role for the differential diagnosis).
Spondylodiscitis (inflammatory endplate lesions, non-corner inflammatory lesions) occurs frequently but has low specificity since degenerative lesions have a similar appearance.
Other inflammatory lesions (facet joint lesions, costovertebral lesions) may be more specific than endplate lesions but are less well studied to date.
There was consensus in the ASAS MRI working group that use of the term ‘Romanus lesion’ in conjunction with MRI-based findings is generally discouraged. One major argument was that, historically, Romanus had described erosions at the corners of the vertebral endplates as detected by conventional x-ray as indicative of AS.21
Structural lesions
Structural changes may present as alteration of the bone marrow, as bony destruction or as bony proliferation. These changes may occur at multiple sites of the vertebral column; the locations are listed in table 2.
The pathology is not described by location but is differentiated by appearance. Structural changes are summarised in table 3.
Types and descriptions of structural changes
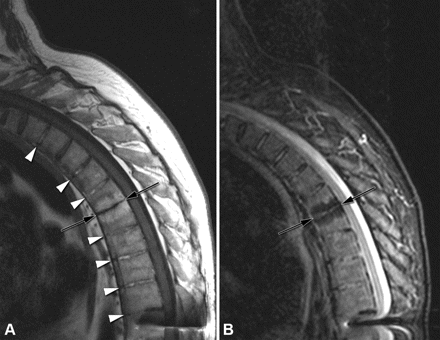
Fatty deposition, erosion and ankylosis. (A) T1 image showing high signal intensity due to fatty deposition predominantly at vertebral corners (white arrowheads) as well as adjacent to endplates of selected vertebral bodies. Erosion (black arrowhead) of lower endplate of T12. Vertebral unit L3/4 shows ankylosis through the intervertebral disc (black arrow) and within the annulus fibrosus (white large arrow). A small syndesmophyte is seen at the upper endplate of L3 (white small arrow). (B) Corresponding STIR image showing residual osteitis at T6, T8, T9 and T10 (white arrows), but not in areas affected by fatty deposition. STIR, short τ inversion recovery.
Ankylosis. (A) T1 image depicting transdiscal ankylosis of several segments from T1/2 through T9/10 (white arrowheads)—see hyperintense signal intensity within intervertebral discs in the respective segments. Vertebral unit T5/6 is spared out and allows minimal segmental motion, resulting in reduced disc height and fatty marrow infiltration of adjacent bone marrow (black arrows). (B) Corresponding STIR image without any inflammation. Hypointense signal at T5/6 (black arrows) due to suppressed fat signal (note: artefacts due to metal screws of spondylodesis at T11 and below). STIR, short τ inversion recovery.
Each of these structural lesions described, also, must be visible in at least two or more consecutive sagittal slices.
Structural lesions: summary
As a result of consensus discussions and incorporating the published evidence,22 ,23 the group agreed on the following statements:
Fatty deposition at vertebral corners is typical for axial SpA.
Detection of fatty deposition at vertebral corners, particularly if present at several sites, increases the likelihood of axial SpA, especially in the younger age group.
Erosions, syndesmophytes and ankylosis are visible on spinal MRIs. However, the final value of MRI with respect to structural changes needs further study.
Literature search
In total, four papers were further analysed after performing a structural literature search as outlined in the Methods section and in figure 1.11 ,20 ,22 ,23 Kim and coworkers evaluated 52 patients with known AS according to the mNY (modified New York) criteria5 and compared them to 52 age- and sex-matched non-AS controls in a retrospective case–control study.22 T1w and T2w MRI sequences without fat suppression were analysed. Corner-related MR changes, the so-called ‘MR corner sign’, were the main focus of this paper (both active changes and structural lesions). The sensitivity and specificity of the MR corner sign were 44% and 96%, respectively.
From Leeds, two papers were recently published. The first one focuses on active changes.11 A total of 174 patients with back pain (64 patients with SpA, 45 patients with degenerative disc disease, 45 patients with malignant spine disease and 20 patients with other diagnoses) as well as 11 healthy controls were included. T1w and STIR sequences of the entire spine were acquired. The evidence of three or more anterior or posterior spondylitic lesions resulted in a specificity for SpA of 81% and a positive likelihood ratio (LR) of 2.5. When age was taken into account (≤50 years), a specificity of 97% and a positive LR of 12.5 were calculated. A second evaluation of the same patients had a focus on fatty degeneration at the corners of vertebral bodies.23 Clearly, these fatty bone marrow changes occur in 31% of patients with SpA but in only 13% and 4% in the groups of patients with degenerative disc disease and malignancy, respectively. This results in a specificity of 93% and a positive LR of 4.7 if only one lesion is present and increases to 98% and a positive LR of 12.6 if five or more corner-related lesions of fatty bone marrow degeneration are present.
Weber and coworkers analysed STIR images of the entire spine of 35 patients with AS with a Bath Ankylosing Spondylitis Activity Index of ≥4.0 and compared them to STIR images of 35 age- and sex-matched healthy controls.20 In addition, 27 patients with inflammatory back pain (IBP) not fulfilling the mNY criteria were investigated. In contrast to the other studies, three readers evaluated the images independently and a lesion was only counted in the statistical analysis if at least two of the three readers detected it. In this study, inflammatory spondylitic lesions occurred in 26% of healthy controls. The presence of two or more spondylitic lesions resulted in a specificity of 94% with a positive LR of 12.0 in the AS group and 96% and 8.0, respectively, in the IBP group.
To summarise the literature review, it appears that corner-based lesions (either inflammatory or structural) are typical for axial SpA but can also occur in other diseases. However, spinal inflammation in axial SpA does occur at different anatomical locations, which may have broad overlap with other diseases (eg, intervertebral discs) or are currently less well studied (eg, costovertebral and zygoapophyseal joints). Detection of two (or three, depending on study population and study design) or more spondylitis lesions in the age group below 50 years increases the likelihood of having axial SpA. Detection of multiple structural (fatty) corner-based lesions increases the likelihood of having axial SpA, especially in the younger age group. One proposal is ≥4 such lesions. However, more data are needed and further studies are required. To the end, all four studies have limitations—therefore, a prospective study in patients under the age of 45 presenting with back pain should be preferentially performed.
Differential diagnoses
There are three patterns of spinal changes due to axial SpA seen on vertebral bodies or units that can be assigned to three different MRI appearances:
bone marrow oedema and hyperaemia during the inflammatory phase
fatty bone marrow degeneration caused by esterification of fatty acids24
sclerosis and ossification.
Mixed appearances may be seen in more advanced disease stages in patients with exacerbation of inflammatory disease. Especially the structural lesions at the discovertebral interface associated with SpA must be differentiated from non-rheumatic changes, which may have a similar appearance on MRI.25
Degenerative disc disease is a frequent cause of chronic back pain. Modic et al described the MRI appearance of the vertebral endplates and adjacent bone marrow in the presence of degenerative changes of the intervertebral disc.26 Based on their signal patterns, three types of lesions were distinguished by Modic et al. Histopathological examinations of type 1 lesions demonstrated fissures of the vertebral endplates and vascularised connective tissue. Type 2 lesions have been histopathologically correlated with fatty degeneration of the bone marrow. ‘MR corner signs’, either by inflammation or with postinflammatory fatty degeneration, may also be visible in degenerative spondylophytes. Disc degeneration is associated with narrowing of the intervertebral space and ingrowth of fibrovascular tissue with subsequent oedema formation in the bone marrow of adjacent vertebral bodies, eventually in conjunction with erosions.27
Disc herniation into the spongiosa of patients with juvenile kyphosis (Scheuermann's disease) is characterised by similar signal changes on MRI: inflammatory signals in and around Schmorl's nodules have been regarded as signs of activity, which may later undergo fatty degeneration and finally sclerosis.28
Infectious spondylitis is an acute vertebral osteomyelitis caused by haematogenous, or less commonly, exogenous (perioperative), spread of pathogens into vertebral bodies.29 A special feature of infectious spondylitis is the high frequency of infectious inflammatory foci or abscesses located pre- and/or paravertebrally, intraspinally, and/or epidurally.30 MRI depicts active infectious spondylitis with a low signal intensity on T1w images while T2w sequences and the STIR sequence document the involvement of affected vertebrae and discs by a high signal. A T1w/Gd sequence is usually needed to diagnose abscesses.
Spinal ossifications in diffuse idiopathic skeletal hyperostosis (DISH) can simulate AS.31 However, the morphology of the ossifications differs and can usually be differentiated on conventional radiographs. While the ossification of the outer parts of the annulus fibrosus associated with AS develops in a vertical direction, DISH is characterised by more voluminous multisegmental ossifications of the anterior longitudinal ligament with preservation of the height of the intervertebral spaces.32 ,33
Image examples of the differential diagnoses described here are given in the ASAS handbook.34
Discussion
This consensus paper spells out the agreement process of our international ASAS/OMERACT expert group consisting of rheumatologists and radiologists. The spine plays a major role in MRI-based scoring systems, which have been developed for the evaluation of treatment effects in clinical studies, and in particular patients with long-standing and established AS show signs of inflammation in the spine rather than in the SI joints, which are affected during the early disease stages.8 ,11 ,35
Spinal MRI is currently considered a powerful tool to document treatment effects since improvement, persistence or new onset of spinal inflammation in AS can be monitored.36 ,37
Furthermore, spinal inflammation on MRI is now increasingly used as a tool to select patients for anti-tumour necrosis factor (TNF) treatment or to predict the effect of anti-TNFα treatment.38,–,40
The work presented here reflects an important consensus process that has become necessary because only opinion-based review articles of individual authors had been published on this topic to date.15 ,25 ,41 ,42
The definitions developed in this process are simple and easy to apply. All inflammatory lesions share common features in terms of signal characteristics on T1w and STIR sequences. These lesions may be differentiated based on their location in the spine.
Spinal MRI so far has been mainly used to detect and localise inflammatory changes,2 ,42 ,43 but two studies have suggested that structural changes as detected by MRI may also be useful signs for classification and diagnosis.11 ,14
Structural lesions are characteristic for more advanced chronic stages of axial SpA. How much time is needed until structural lesions (eg, fatty degeneration of the bone marrow) develop is unknown. However, there is limited evidence that between 6 months and 2 years have to elapse until an inflammatory lesion transforms into fatty bone marrow.11 ,44 Some debate exists in the scientific community, whether fatty degeneration of bone marrow is a true structural change. Indeed, the recent scientific discussion focuses on fatty lesions, and they seem to be the connecting link between inflammation and true structural damage (as we know it from conventional x-ray). Fatty lesions as such seem to be an irreversible change of the bone marrow; therefore, it seems to be justified to list them under structural changes rather than inflammation.
The structured literature search as part of the process outlined in this paper was performed to back up the results of the expert discussions. It was felt that many articles already describe the different lesions in SpA, but the majority of papers are review-type articles that only express single expert opinions. As new data were published during the discussion process, the group deemed it necessary to readjust the results based on these data. Interestingly enough, the results of the controlled studies selected for an in-depth analysis were in concordance with the expert opinions.
The experts agreed on the statement that no single lesion described in this paper is specific for axial SpA. However, current data suggest that a combination of inflammatory lesions11 ,20 or a combination of structural lesions23 provides sufficient specificity to make a diagnosis of axial SpA based on a spinal MRI. The experts found it premature to propose a number as a cut-off at this point in time.
The experts agreed to state that there are several different diseases manifesting in the spine that may show similar patterns on MRI. The degree of the MRI changes seen in these entities clearly depends on the stage of the respective disease. This is especially true for diseases such as juvenile kyphosis (Scheuermann's disease), septic spondylodiscitis, erosive osteochondrosis and other degenerative disc diseases.
In SpA, bone marrow oedema plays a major role in the definition of active disease in the spine. In long-standing disease, a correlation of bone marrow oedema by MRI at apophyseal joints with the histological degree of inflammation has been found.45 Similar results have been described for the SI joints.46
Definitions of the different spinal lesions described here by the ASAS MRI working group were also recently described by the Canada–Denmark MRI working group.47 ,48 The acronymic abbreviations of these lesions were incorporated in the tables of the current publication in order to demonstrate that, in principle, the definitions of this nomenclature and the descriptions of the ASAS/OMERACT MRI working group are the same.
The results of this work were presented in detail to the ASAS members in January 2010 at the annual ASAS assembly, were discussed and refined and were approved by a final vote. By using this approach, we aimed to integrate the available worldwide expertise on this topic. Nevertheless, these definitions should be revised if necessary based on newly available data. The next step will be the validation of these definitions in clinical research. A common language makes a comparison across studies possible. It will also be important to define the validity of the definitions in discriminating patients with SpA from healthy persons and individuals with other diseases.
References
Supplementary materials
Web Only Data
Files in this Data Supplement:
Footnotes
Competing interest None.
Provenance and peer review Not commissioned; externally peer reviewed.