Article Text
Abstract
Objectives To investigate the prevalence of osteoarthritis (OA) in patients with diabetes mellitus (DM) and prevalence of DM in patients with OA and whether OA and DM are associated.
Design A systematic literature review and meta-analysis. We included cohort, case–control and cross-sectional studies assessing the number of patients with DM and/or OA. The mean prevalence of OA among patients with DM and DM among patients with OA was calculated. Data from trials assessing an association of diabetes and OA were pooled and results are presented as unadjusted OR and 95% CI.
Results From the 299 publications, we included 49 studies in the analysis, including 28 cross-sectional studies, 11 cohort studies and 10 case–control studies. In all, 21, 5 and 23 articles involved patients with OA exclusively, patients with DM and the general population, respectively. For 5788 patients with DM, the mean OA prevalence was 29.5±1.2%. For 645 089 patients with OA, the prevalence of DM was 14.4±0.1%. The risk of OA was greater in the DM than non-DM population (OR=1.46 (1.08 to 1.96), p=0.01), as was DM in the OA than non-OA population (OR=1.41 (1.21 to 1.65), p<0.00 001). Among the 12 studies reporting an OR adjusted on at least the body mass index, 5 showed no association of DM and OA and 7 identified DM as an independent risk factor.
Conclusions This meta-analysis highlights a high frequency of OA in patients with DM and an association between both diseases, representing a further step towards the individualisation of DM-related OA within a metabolic OA phenotype.
- Osteoarthritis
- Epidemiology
- Hand Osteoarthritis
This is an Open Access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Statistics from Altmetric.com
Key messages
What is already known on this subject?
Metabolic syndrome and osteoarthritis have been found to be associated in some studies, delineating the metabolic osteoarthritis phenotype. Association between diabetes mellitus and osteoarthritis in epidemiological studies have given conflicting results.
What does this study add?
This is the first meta-analysis showing an association between osteoarthritis and diabetes mellitus.
How might this impact on clinical practice?
Treating diabetes mellitus may be effective in patients with osteoarthritis.
Prevention initiatives of osteoarthritis may be specifically proposed to patients with diabetes mellitus.
Introduction
Osteoarthritis (OA) is the most frequent and disabling joint disease in adults. Besides its several localisations, a recent hypothesis has suggested a new classification for phenotyping OA that includes ageing, metabolic syndrome (MetS) and post-traumatic events and genetic-related OA.1 ,2 Despite some shared pathophysiological mechanisms among these phenotypes, each may display specific pathways.
In MetS-associated OA, the mechanical impact of overweight or obesity on joints may easily explain knee OA. However, within this phenotype, considering the epidemiological association of overweight or obesity and hand OA, some systemic factors may participate in the pathogenic process; for example, adipose tissue products, or ‘adipokines’, may have a systemic impact at a distance on joints.3–5 Beyond obesity-related OA, MetS and OA have been found to be associated in some epidemiological studies, which suggests that the other components of MetS, such as diabetes mellitus (DM), high blood pressure or dyslipidaemia may together or independently participate in the OA pathophysiology.6 ,7 Along this line, DM and hyperglycaemia seemed to be associated with OA in some epidemiological studies.8–11 Moreover, the link between the two diseases may be supported by the deleterious role of glucose excess through the accumulation of advanced glycation end products (AGEs), oxidative stress and promotion of systemic inflammation.12–15 This situation is well illustrated by spontaneous cartilage disruption in the rat model of streptozotocin-induced diabetes.12–15 However, other publications have questioned the link between DM and OA.16 ,17
To further address the association of OA and DM, we performed a systematic review of the literature and a meta-analysis to investigate the prevalence of OA among patients with DM and that of DM among patients with OA and to determine whether DM and OA are associated.
Methods
Systematic literature search and selection of relevant studies
We performed a systematic review of the literature according to the Cochrane guidelines (http://handbook.cochrane.org/, 24 February 2014, date last accessed). Relevant publications were selected from three databases (PubMed, EMBASE and the Cochrane Library) without any limitation on time (up to June 2013 and updated in January 2015). We also searched for articles in the references of selected publications and the main congresses of rheumatology for OA (American College of Rheumatology (ACR), European League Against Rheumatism (EULAR) and Osteoarthritis Research Society International (OARSI)) and congresses of endocrinology (Endocrine society's annual meeting, European Congress of Endocrinology, American Diabetes Association and European Association for the Study of Diabetes) from 2012 to 2014. We used the following key words for the PubMed search: (“diabetes mellitus, type 2”[MeSH] OR “diabetes mellitus, type 1”[MeSH] OR “diabetes complications”[MeSH] OR “metabolic syndrome X”[MeSH] OR (“blood glucose”[MeSH] OR “blood glucose”[All Fields])) AND “osteoarthritis”[MeSH] AND (“humans”[MeSH] AND (English[lang] OR French[lang])).
We included observational studies (ie, cohort, case–control and cross-sectional studies) assessing the number of patients with OA and/or DM, or the incidence or prevalence of OA in patients with DM or DM in patients with OA, or an association between OA and DM. We excluded articles of therapeutic studies, reviews and case reports as well as letters; studies in which all patients had OA and DM or in which the link between OA and DM was not reported; and studies without an available number of patients with each disease. Selection of articles was based on titles and abstracts than on the full text. One author (KL) has managed this selection.
Data extraction
Two authors (KL and CV) extracted the following data: study design and population (observational study, quality score, definition of DM and OA and localisation of OA); exposure glycaemia (fasting blood glucose (FBG) level, mmol/L) or glycosylated haemoglobin (HbA1c; %) or number of patients with DM; outcome (number of patients with OA); body mass index (BMI, kg/m2) or number of patients with obesity as potential confounders; association measure (relative risk or OR or only conclusion on an association). Then we conversely considered OA as an exposure factor and DM as an outcome. The study quality was assessed by the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) scale and results are reported as a percentage of 19 pertinent items of the 22 total items.18
Statistical analysis
We performed two analyses with available results from trials. First, we performed a descriptive analysis: for cross-sectional, case–control or cohort studies, we used the number of patients with OA or DM and total number in each population to calculate the prevalence of OA among patients with DM and that of DM among patients with OA. To estimate this prevalence from cohort longitudinal prospective studies, we used baseline data. Prevalence is expressed as mean±SD. Second, we performed a comparative analysis using studies assessing an association between diagnosis of DM and OA in cross-sectional studies and cohort studies of the general population and case–control studies of OA or DM populations. We calculated the odds of having OA among patients with DM and of DM among patients with OA with ORs and 95% CIs. Then we used Revman V.5.2 to perform a meta-analysis with a fixed-effects model. A random-effects model was used with high heterogeneity among studies (>50%), evaluated by I2, and a sensitivity analysis was performed by removing studies with aberrant results and combining studies with the same characteristics. OR>1 and p≤0.05 was considered an increased risk of OA among patients with DM and/or DM among patients with OA.
Results
Literature search and characteristics of included trials
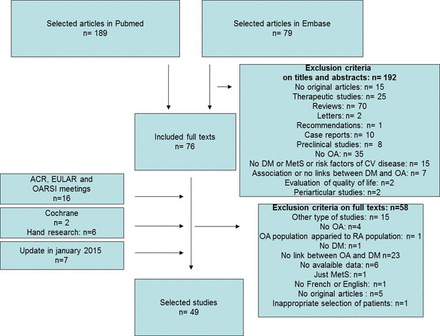
The selection of articles is in figure 1: from 299 publications, we included 49 in the analysis. We found no publication bias (see online supplementary figure S1). The articles represented 28 cross-sectional, 11 cohort and 10 case–control studies. In total, 21, 5 and 23 involved exclusively patients with OA, exclusively patients with DM and the general population, respectively (table 1).
Description of the 49 studies selected for analysis
Flow chart of selection of articles. OA, osteoarthritis; DM, diabetes mellitus; RA, rheumatoid arthritis; MetS, metabolic syndrome; CV, cardiovascular; ACR, American College of Rheumatology; EULAR, The European League Against Rheumatism; OARSI, Osteoarthritis Research Society International.
The criteria of inclusion for the general population were variable for age: from 20 to 86 years for the Iwaki Health Promotion Project,20 and from 65 to 84 years for the ILSA study;46 the patients had radiographs of hips for the study of Typpo,31 or data were extracted from public service data for the study of Navarro et al.34 In most cases, OA was defined by radiological and clinical criteria, usually based on the ACR criteria. DM was defined by elevated glycaemia, HbA1c proportion or prescription of DM treatment (table 2).
Characteristics of the 49 included studies
Only two studies specified the number of patients with type 1 and type 2 DM:19 ,57 in the study of Nieves-Plaza et al,57 there were only 14 patients with type 1 DM with a similar repartition in the OA and non-OA groups, and in the study of Ray et al19 there were 11% patients with type 1 DM with a similar repartition in the OA and non-OA groups. The median STROBE quality score was 69% (range 33–91%; table 2). For 6 studies (4 case–control and 2 cross-sectional studies), the score was <50%, and for 30 studies it was >60%. The country of origin of the studies was diverse (22 studies with patients from Europe and 16 studies with patients from North America). For four studies, OA was severe because the outcome was arthroplasty. Among the 49 studies, 34 assessed the association between OA and DM, 28 the frequency of DM among patients with OA and 24 the frequency of OA among patients with DM.
Characteristics of patients
A total of 1 192 518 patients were included in the analyses. The mean age ranged from 43.8±43.9 to 76.9±5.4 years.7 ,46 The mean proportion of females was 78.92% (from 9.3% to 100%).29 ,35 ,63 The localisation was the knee for 31 studies (knee only for 13 studies), the hip for 15 studies (hip only for 3 studies), hands for 12 studies (hands only for 4 studies) and the spine for 5 studies (see online supplementary table S1). The mean FBG level ranged from 3.95 (no SD available) to 12.17±6.49 mmol/L and HbA1c from 5.1±0.1% to 7.2% (no SD available).19 ,20 ,63 ,64 The prevalence of obesity varied greatly from 9.1% to 73.2%, and BMI ranged from 22.3±2.7 to 33.8±5.8 g/cm2.7 ,20 ,34 ,35 ,36 MetS was reported in five studies using different definitions in which hyperglycaemia was one of the items and involved 5.1–58.6% of patients.7 ,20 ,34 ,36 ,42 ,48
Prevalence of OA among patients with DM and DM among patients with OA
For 5788 patients with DM, the mean OA prevalence was 29.5±1.2% (mean age=61.01 years). This prevalence was calculated by using the 5 and 12 studies of patients with DM and the general population, respectively, with available data on the number of patients with OA in the DM population (see online supplementary table S2). In this population, the prevalence of OA calculated with available data for each localisation was 17.2±2.0% for the knee,10 ,19 ,20 ,34 ,42 ,48 12.3±1.3% for the hip31 ,48 and 38.4±6.8% for the hand.36 ,50
For 645 089 patients with OA, the DM prevalence was 14.4±0.1%. It was calculated by using the 19 and 12 studies of patients with OA and the general population, respectively, with available data on the number of patients with DM in the OA population (see online supplementary table S2). Three studies involving patients with OA were not included because they assessed semiquantitative or continuous variables, the Kellgren-Lawrence (KL) score and glycaemia but not OA or DM diagnosis.56 ,60 ,63
Associations between OA and DM
In total, 34 studies assessed the association of OA and DM and/or glycaemia or HbA1c proportion; 21 showed a significant association in their conclusions or at least reported OR>1 in the text,7 ,8 ,10 ,20 ,25 ,28 ,29 ,39 ,41 ,44 ,46 ,50–52 ,54 ,55 ,57 ,58 ,60 ,61 ,63 whereas 12 studies displayed no association.16 ,17 ,27 ,31 ,34 ,38 ,42 ,48 ,49
Risk of OA in DM: meta-analysis and sensitivity analyses
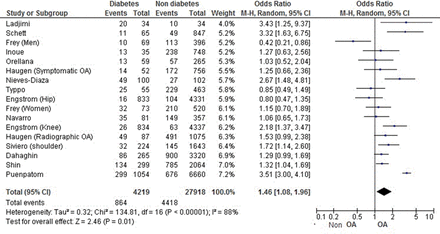
For risk of OA in a DM versus non-DM population, among 32 137 patients, the overall OR was 1.46 (1.08 to 1.96), with high heterogeneity (I2=88%; figure 2). After excluding poor-quality studies (ie, STROBE score <50%), the heterogeneity did not change (I2=88%).55 ,31 Considering only studies with validated criteria for diabetes including glycaemia or HbA1c and excluding studies with declarative data only, the OR was 1.58 (1.14 to 2.20), with similar heterogeneity (I2=89%). Considering all studies with the same design (ie, cross-sectional, cohort or case–control studies), the OR was significant for only case–control studies (2.85 (1.71 to 4.73); I2=0%).
Forest plot for osteoarthritis among patients with and without diabetes mellitus.
Among OA risk factors, age and obesity have a strong impact on OA development. Considering only studies with patients ≥50 years old, the OR was 1.32 (1.13 to 1.53), without heterogeneity, which suggested that the association remained even in patients at increased risk of OA because of their age.25 ,34 ,36 ,42 ,46
Among the 12 studies with OR adjusted on BMI, 5 showed no association between DM and OA,16 ,25 ,27 ,48 ,49 but 7 identified DM as an independent risk factor of OA,8 ,10 ,29 ,44 ,46 ,51 ,57 and were of higher quality as illustrated by the mean STROBE scale (69±7.4% vs 76±8.5%, respectively). Interestingly, the positive studies were the recent ones: six of seven were published between 2009 and 2013.8 ,10 ,44 ,46 ,51 ,57 Considering OA localisations: the results were significant for knee OA-only and hand OA-only (OR=1.64 (1.17 to 2.29) with 9170 patients10 ,20 ,34 ,42 ,48 ,55 and OR=1.31(1.07 to 1.61) with 5879 patients,25 ,36 ,50 respectively) but not for hip (OR=0.82 (0.56 to 1.21), with 5682 patients).31 ,48
Risk of DM in OA: meta-analysis and sensitivity analyses
For risk of DM in an OA versus non-OA population, among 1 040 175 patients, the overall OR was 1.41 (1.21 to 1.65), assessed by a random-effects model because of I2=95% (figure 3).
Forest Plot for DM among patients with and without OA (OA, osteoarthritis; DM, diabetes mellitus).
We performed four sensitivity analyses to strengthen the results. First, with the heterogeneity explained by two studies with aberrant results, we removed these two studies.54 ,59 The OR remained similar: 1.42 (1.22 to 1.66), I2=96%. Second, we focused on severe OA (ie, the 3 studies with surgery as an OA outcome corresponding to 11 805 patients); the OR was not significant: 1.32 (0.52 to 3.36) but with high heterogeneity (I2=85%).10 ,48 ,59 Third, we removed the studies that did not use internationally recognised diagnosis criteria for OA such as ACR criteria or the KL score for OA definition; the OR was 1.32 (1.13 to 1.53) without any heterogeneity (I2=0%) with five studies and 9947 patients.20 ,25 ,42 ,50 ,58 Fourth, we considered OA localisations: for data involving knee OA only or hip OA only, the results were significant for the knee (OR=1.51 (1.09 to 2.09) with 5 studies and 9102 patients) but not for the hip (OR=0.71 (0.49 to 1.04) with 3 studies and 6240 patients).10 ,20 ,31 ,34 ,42 ,48 ,59 We also found a significant association for non-weight-bearing hand OA (OR=1.31 (1.07 to 1.61)). There was no study that included only generalised OA.
Discussion
OA is a heterogeneous disorder that can be separated in an age-related, metabolic and post-traumatic OA, representing thus the three main phenotypes of the disease. Metabolic OA is wider than obesity-related OA since MetS and OA are epidemiologically linked.2 ,7 However, the association between each component of the MetS and OA needs to be further addressed. Likewise, we aimed to assess the overall link between OA and DM. We performed a systematic review of the literature and meta-analysis of data from 49 studies involving a large sample of participants (n=1 192 518). The prevalence of OA among patients with DM was 29.5±1.2% and that of DM among patients with OA was 14.4±0.1%. Moreover, OA and DM were significantly associated: the overall risk of OA in the DM population was 1.46 (1.08 to 1.96) and that of DM in the OA population was 1.41 (1.21 to 1.65).
In the DM population, the risk of OA was significant with overall data. All studies had approximately the same weight in the analysis. Such a result was confirmed in patients older than 50 years: DM seems to be associated with OA, even when age may have a significant impact on OA, which suggests that the link with DM does not depend on age.
In the OA population, the risk of DM was also significant (OR=1.41 (1.21 to 1.65)). Data from two studies had an important weight on this finding: DM prevalence in the OA group was 9.7% in the study of Rahman et al,61 and 9.8% in the work of Wang et al62 coming from a congress abstract, and the definitive publication for this abstract will be critical to confirm these results. It can influence the final outcome, but the Rahman et al61 study was of good quality. The association of OA and DM was not significant when we considered only studies of severe OA (ie, time to joint replacement), probably because of the small number of patients.10 ,48 ,59 The role of DM in progression of OA is controversial since Yoshimura et al64 have found that DM defined as HbA1c fraction ≥5.5% was not independently associated with OA progression, whereas in a recent study type 2 DM was a significant predictor of joint space narrowing in males with symptomatic knee OA.65
In addition, recruitment bias at the time of joint replacement may explain the findings because the presence of comorbidities such as DM may restrict the indication for surgery in terms of a potential increase in subsequent perioperative adverse events. We found an especially significant association between DM and OA with the studies including hand OA only, which highlights the metabolic and systemic nature of hand OA, highlighted recently in the NEO cohort.53 ,66 Moreover, the impact of DM on symptoms or on structural lesions might be different. Schett et al67 have shown that symptoms of OA assessed using the Knee injury and Osteoarthritis Outcome Score (KOOS) and the Western Ontario and McMaster Universities Arthritis Index (WOMAC) were more severe and ultrasound synovitis and effusion of knees more frequent in participants with type 2 DM than those without DM. This inflammatory aspect in imaging corroborates with the higher release of inflammatory mediators in OA cartilage explants from patients with DM than those from patients without DM.68 The well control of DM by antidiabetic therapies could also influence the prevalence of OA: HbA1c fraction, that reflects the three last months of DM control, was significantly higher in women knee OA for Yoshimura et al8 ,64 and for Inoue et al.20 However, we had no data about the history of the DM control during the previous years during which OA developed and progressed. Moreover, data about antidiabetic drugs were used only to identify patients with DM, but not as a factor able to influence OA occurrence or progression. The assessment of the radiographic patterns of DM-related OA (ie, erosive OA, diffuse idiopathic skeletal hyperostosis) was not possible due to a lack of data. The prevalence of OA in patients with DM was 14.4±0.1%. We measured prevalence of DM among patients with OA, emphasising the link between both diseases. This prevalence could be compared with the prevalence of OA in the general population, but it depends on the reference population: 32% of OA in Iwaki Health Promotion Project (Japan) and 13% of OA in NHANES III (USA).7 ,20 We lack basic data on the impact of OA on DM, but this remains to be investigated because of the possible systemic effects of OA.69
Our meta-analysis has some limitations. The heterogeneity was high in the first analyses, probably because of population characteristics (various OA localisations or definitions, various DM definitions, no stratification on DM severity or treatment) or various types and qualities of studies. However, we performed several sensitivity analyses, which allowed a decrease in the heterogeneity level, in particular in subgroups with a well-recognised definition of inclusion criteria of OA. To further decrease heterogeneity, we eliminated some studies: those that were of low quality or with a diagnosis of OA not based on ACR criteria or KL grading.39 ,41 ,54 ,60 However, the heterogeneity and results did not greatly change because of the low weight of these studies in the meta-analysis. Another limitation is the impact of confounding factors, especially age and obesity. However, despite a significant impact of increasing age on OA, the association remained positive when we retained studies including patients older than 50 years. Moreover, we identified seven studies showing an association even after adjustment on BMI in their multivariate logistic regression. The mean score of quality was better and most were recent (results published after 2009). Confounding factors such as joint injury, physical activity, smoking, hypertension, dyslipidaemia might have affected our findings, but these factors were taken into account in each included study.8 ,10 In patients with DM, neuropathy may also affect OA development, but we did not find this information in the selected studies.
We have shown an association of DM and OA, but causality is not yet clearly demonstrated. Hyperglycaemia could promote joint inflammation and cartilage degradation through oxidative stress and inflammatory mediators induction as well as through AGEs.12 Beyond a chronic excess of glucose, type 2 DM is characterised by increased insulin resistance that may be involved in osteophyte development and subchondral bone sclerosis.9 ,70 ,71 We thus need additional specific prospective studies for that purpose.
In summary, this is the first meta-analysis showing an association of OA and DM, giving some additional clues about the delineation of the metabolic OA phenotype. Large prospective studies are needed to address whether DM is an independent risk factor of OA development or severity. If this is the case, new preventive and/or curative modalities based on glycaemia control could be tested in OA.
Acknowledgments
The authors thank Laura SMALES (BioMed Editing, Toronto, Canada) for editing the manuscript. KL, FB and JS are supported by The Foundation Arthritis Network Program (ROAD project).
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
- Data supplement 1 - Online supplement
Footnotes
Contributors KL, JS and FB were involved in conception and design. KL was involved in acquisition of data and statistical analysis. KL, CV, JS and FB were involved in analyses and interpretation of data, drafting of the manuscript, revision of the manuscript and final approval. KL, JS and FB had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Competing interests None.
Provenance and peer review Not commissioned; externally peer reviewed.
Data sharing statement No additional data are available.