Article Text
Abstract
Background Multiple biologic and targeted synthetic disease-modifying rheumatic drugs (b/tsDMARDs) are approved for the management of rheumatoid arthritis (RA), including TNF inhibitors (TNFi), bDMARDs with other modes of action (bDMARD-OMA) and Janus kinase inhibitors (JAKi). Combination of b/tsDMARDs with conventional synthetic DMARDs (csDMARDs) is recommended, yet monotherapy is common in practice.
Objective To compare drug maintenance and clinical effectiveness of three alternative treatment options for RA management.
Methods This observational cohort study was nested within the Swiss RA Registry. TNFi, bDMARD-OMA (abatacept or anti-IL6 agents) or the JAKi tofacitinib (Tofa) initiated in adult RA patients were included. The primary outcome was overall drug retention. We further analysed secondary effectiveness outcomes and whether concomitant csDMARDs modified effectiveness, adjusting for potential confounding factors.
Results 4023 treatment courses of 2600 patients were included, 1862 on TNFi, 1355 on bDMARD-OMA and 806 on Tofa. TNFi was more frequently used as a first b/tsDMARDs, at a younger age and with shorter disease duration. Overall drug maintenance was significantly lower with TNFi compared with Tofa [HR 1.29 (95% CI 1.14 to 1.47)], but similar between bDMARD-OMA and Tofa [HR 1.09 (95% CI 0.96 to 1.24)]. TNFi maintenance was decreased when prescribed without concomitant csDMARDs [HR: 1.27 (95% CI 1.08 to 1.49)], while no difference was observed for bDMARD-OMA or Tofa maintenance with respect to concomitant csDMARDs.
Conclusion Tofa drug maintenance was comparable with bDMARDs-OMA and somewhat higher than TNFi. Concomitant csDMARDs appear to be required for optimal effectiveness of TNFi, but not for bDMARD-OMA or Tofa.
- rheumatoid arthritis
- biological therapies
- tocilizumab
- abatacept
- tumour necrosis alpha inhibitors
- comparative effectiveness research
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
- rheumatoid arthritis
- biological therapies
- tocilizumab
- abatacept
- tumour necrosis alpha inhibitors
- comparative effectiveness research
Key messages
What is already known about this subject?
Only a few of available biologic and targeted synthetic disease-modifying rheumatic drugs (b/tsDMARDs) in RA have been compared directly in trials and the ‘real- world’ effectiveness of the newer Janus kinase inhibitor (JAKi) class compared with the established bDMARDs is still largely unknown.
What does this study add?
The effectiveness of three alternative treatment options for RA, including a JAKi, was compared in a large observational, population-based cohort. The overall drug maintenance for tofacitinib (Tofa) was significantly higher than for TNF inhibitors (TNFi) and similar to biologics with another mode of action (bDMARDs-OMA).
Concomitant therapy with conventional synthetic antirheumatic agents (csDMARDs) did not improve the drug -maintenance of bDMARD-OMA or of Tofa, while TNFi appeared to require comedication with csDMARDs for optimal treatment results.
How might this impact on clinical practice or future developments?
Our results confirm that JAKi, represented here by Tofa, are a valuable alternative to available treatment options in RA, with good ‘real-world’ effectiveness outcomes. Our results also suggest that the relative benefit of concomitant csDMARDs varies according to the type of b/tsDMARD.
BACKGROUND
In the past decades, the management of rheumatoid arthritis (RA) has been revolutionised by new antirheumatic therapies (DMARDs) and by new treatment paradigms, such as the ‘treat to target’ model or the timely initiation of DMARDs in early disease. Among the DMARDs, TNF inhibitors (TNFi) first became available in the late 1990s, followed by biologic DMARDs (bDMARDs) with other modes of action (bDMARD-OMA) such as rituximab, tocilizumab and abatacept during the last decade. More recently, Janus kinase inhibitors (JAKi), such as tofacitinib (Tofa) or baricitinib, were approved for the treatment of moderate-to-severe RA patients having failed methotrexate (MTX). Rheumatologists and their RA patients need to choose between DMARDs licensed with similar indications, including a number of TNFi, several bDMARD-OMA and JAKi. In the management of RA, this has created a unique situation of a relative abundance of targeted therapies for a limited market segment. Unfortunately, no reliable predictors enable to select a specific therapy for a particular patient, leaving the treating physician with few clues for rational choice of the therapy most likely to succeed for a given patient.
Evidence-based treatment decisions involve comparisons of available therapies. Only a couple of randomised trials have compared two of the available treatment options directly and few observational studies have compared simultaneously all alternative therapeutic options, including the newer JAKi class. Registries provide a unique opportunity to explore the clinical effectiveness of therapies in specific clinical situations and distinct patient subgroups, which is becoming more important as we move towards more personalised clinical care. Observational research has demonstrated that seropositive RA patients are more likely to respond well to rituximab or to abatacept.1 Furthermore, analyses of RA registries have suggested that the benefit of concomitant conventional synthetic antirheumatic agents (csDMARDs), such as MTX, may differ among targeted therapies.2 3 In randomised trials, TNFi have generally demonstrated better efficacy together with concomitant MTX or other conventional DMARDs, while tocilizumab or JAKi have not displayed such a strong benefit from cotherapy.4
The aim of this study was to compare the drug maintenance and the real-world effectiveness of three alternative treatment options, licensed with a similar indication, namely TNFi, bDMARD-OMA and Tofa, using data from a Swiss Registry. We further aimed to examine whether the effectiveness of these agents was modified by concomitant csDMARD therapy.
PATIENTS AND METHODS
Study design and population
We performed this nested cohort study within a prospective, longitudinal registry of patients with RA, the Swiss Clinical Quality Management in Rheumatoid Arthritis (SCQM-RA). The SCQM-RA-cohort can be considered a true population-based sample in terms of targeted antirheumatic therapies. No binding treatment guidelines exist in Switzerland, and access to these therapies is relatively liberal. More than half of the patients are enrolled in the SCQM by rheumatologists in private practice, reflecting the heavily practice-based medical system for rheumatologic care of the country. The registry has been described in detail elsewhere.5 Briefly, patients are enrolled and followed-up one to four times yearly by their treating rheumatologist. The clinical information is collected by the treating rheumatologist and the patient, including disease activity, safety outcomes, medication use and patient-reported outcomes. The SCQM Register has been approved by a national review board, and all participants gave informed consent before enrolment, in accordance with the Declaration of Helsinki.
Exposure of interest
The primary exposure of interest for this analysis was the type of b/tsDMARD used, namely TNFi, bDMARD-OMA or JAKi. The TNFi group included patients initiating any of the following agents: adalimumab, certolizumab pegol, etanercept, golimumab or infliximab, including available biosimilars of some of these TNFi. The OMA-bDMARD group included patients initiating abatacept, sarilumab or tocilizumab. We excluded a priori from this analysis patients starting rituximab, because of the difficulty to define treatment discontinuation with this agent in an observational setting. The oral JAKi tofacitinib (Tofa) was licensed in Switzerland in 2013 for the treatment of moderate-to-severe RA patients having failed MTX. Baricitinib, a second JAK-inhibitor, was approved in Switzerland only in September 2017, resulting in insufficient data for this agent to reliably analyse its effect at the time of this analysis. Thus, we decided to focus on Tofa for the JAKi group.
We planned to analyse effect modification by concomitant csDMARDs, in order to explore whether the relative effectiveness of TNFi, bDMARD-OMA or Tofa is altered by concomitant csDMARDs. B/tsDMARD was categorised as combination therapy (COMBO) if a csDMARD was ongoing at or started within 30 days of the treatment initiation. Combination therapy could be with MTX, leflunomide, sulfasalazin, azathioprine and hydroxychloroquine, alone or in combination. If the b/tsDMARD was started in the absence of a csDMARD, it was categorised as monotherapy (MONO).
Study outcomes
The primary outcome of this study was the overall drug maintenance (‘drug survival’ or ‘drug retention rate’). Drug maintenance integrates both the effectiveness and the tolerance of a drug and is reliably assessed in observational registries, such as the SCQM. We defined ‘time on drug’ as the period between treatment initiation and treatment discontinuation, plus one dispensation interval. For treatments without an observed stop, drug maintenance was censored at the last database entry of the patient. More detail is supplied in the supplemental material file.
Supplemental material
Secondary outcomes were response rates of patients in terms of reaching low disease activity (LDA) based on the Clinical Disease Activity Index (CDAI≤10) at 1 year. CDAI remission was a rare outcome in this patient population with longstanding disease and was not analysed. We decided a priori to assess disease activity using the CDAI instead of the DAS28 to avoid an assessment bias in favour of medications with a strong impact on acute-phase reactants.
Confounding, bias and covariates
To minimise the risk of bias by calendar year, we restricted the sample to adult patients (+18 years) starting a new treatment during a period when all three DMARD groups were available in Switzerland. Tofa was approved in Switzerland in August 2013, so we restricted the analysis to all b/tsDMARDs started in the period between August 2013 and September 2019. A dichotomous variable was included in the models to distinguish between the calendar time before and after the launch of the second JAKi, as the availability of a new therapy could have impacted drug maintenance of the other therapies. Another major predictor of treatment retention is line of therapy, which is likely to differ between drug groups. To address this, we planned a priori to adjust the analysis by line of therapy: Bio-naive patients, a single prior b/tsDMARD failure, two prior b/tsDMARD failures or three or more prior b/tsDMARD failures.
The analysis was further adjusted for potential baseline confounders, including various disease characteristics, namely disease activity (DAS28), disease duration, seropositivity (RF or ACPA) and patient characteristics, namely sex, age, tobacco smoking and body mass index (BMI). Baseline was defined as the date of treatment initiation (first dose) for each treatment course (TC). More detail is supplied in the supplemental material file.
Statistical analysis
Baseline disease and patient characteristics were compared between patients starting TNFi, bDMARD-OMA or Tofa therapies, using descriptive statistics. We performed two-sided statistical tests at the 5% significance level including χ2 for categorical and Mann-Whitney U tests for continuous variables.
We used Kaplan-Meier plots to examine drug retention and Cox proportional hazard models to analyse the hazard for treatment discontinuation. The covariates used for the multivariable adjustment are described above. To determine if concomitant csDMARD therapy has a differential impact on the treatment b/tsDMARD groups of interest (TNFi, bDMARD-OMA, Tofa), we included an interaction term between drug type and cotherapy. In order to analyse specific reasons for discontinuation (ie, ineffectiveness and adverse events), we used a competing risk regression performing a cumulative incidence function, using the same adjustment variables. We had insufficient information and too few events to examine specific adverse events by drug.
Response was evaluated by analysing the percentage of treatment courses where a low CDAI (≤10) was reached 1 year (±3 months) after treatment initiation. In this analysis, we only included TCs that were started at least 1 year before database closure of the 31st of September 2019 and followed up for at least 1 year (last visit in the registry at least 1 year after treatment start). Treatments that were discontinued before 1 year or had a CDAI above 10 were considered non-responders.6 In a substantial proportion of TCs, a follow-up assessment of disease activity at 1 year was missing (~69%). We, therefore, used a mixed-effects model to predict the CDAI at 1 year when missing, based on the other available CDAI assessments, adjusting for potential confounders described above. We used a multivariable adjusted logistic regression model to analyse the likelihood of reaching CDAI LDA state at 1 year and derive ORs in the different treatment groups of interest.
We applied multiple imputation with chained equations to account for missing baseline covariate data. We used 75 datasets with 30 iterations each for imputation. Convergence of imputations was assessed by visual inspection of the mean and variance changes by iteration and dataset. The models were fitted to each dataset, and results were combined using Rubin’s rule.7 More details about the statistical methods used are available in an online supplement.
RESULTS
A total of 4023 TCs were initiated during the study period in 2600 patients; 806 initiated Tofa, 1862 TNFi and 1355 a bDMARD-OMA (table 1). A detailed description of the number of patients included and excluded at the different steps of data preparation is available in the supplementary material (Table S2). Some differences in disease and treatment characteristics existed between the three treatment groups, in particular, TNFi tended to be used more often as a first or second b/tsDMARD, resulting on average in younger patients with shorter disease durations (table 1). MTX was by far the most commonly prescribed csDMARD in combination with second-line therapies (>90% of combination therapies were with MTX), without relevant differences in the relative proportion of MTX between the groups. However, the proportion of patients taking their b/tsDMARD as monotherapy varied significantly between TNFi (29% of MONO), bDMARD-OMA (41% of MONO) and Tofa (47% of MONO) (p<0.001). The proportion and the type of concomitant csDMARDs did not vary considerably over the first year of therapy, and most patients starting as COMBO stayed on concomitant csDMARDs at 1 year (91%, 86% and 81%, respectively, for TNFi, bDMARD-OMA, and Tofa, respectively), and conversely, most patients starting as MONO remained without concomitant csDMARDs at 1 year (89%, 92% and 94%, respectively).
Baseline disease and treatment characteristics
Drug retention
Of the 4023 TCs, 2103 treatment discontinuations were reported during a median follow-up time of 3 years (IQR 2 to 6). The crude overall drug retention differed between the three drug groups (p=0.012, log-rank test). The median drug maintenance was 17 months (IQR 15 to 18) for TNFi, 19 months (IQR 17 to 22) for bDMARD-OMA and 25 months (IQR 19 to 30) for Tofa. After adjusting for potential confounding factors, we found a higher hazard of drug discontinuation with TNFi compared with Tofa [HR 1.29 (95% CI 1.14 to 1.47)], though no significant difference was observed between bDMARD-OMA and Tofa [HR 1.09 (95% CI 0.96 to 1.24)] (figure 1A). Other variables significantly associated with drug discontinuation were a higher number of previous bDMARDs, shorter disease duration, higher BMI values and more recent treatment initiations (Supplementary table S3). Drug maintenance significantly decreased over calendar time for all three b/tsDMARDs (p<0.001). The decrease in drug maintenance was most pronounced for TOFA in recent years, in particular since the launch of a competitor JAK inhibitor [HR 1.71 (95% CI 1.28 to 2.28)].
Drug discontinuation for any reason in patients with RA on TNFi, bDMARD-OMA and Tofa. TNFi=TNF inhibitors. OMA=bDMARDs with other modes of action (abatacept and anti–IL6 receptor), Tofa=Tofacitinib. Adjusted survival curves based on 4023 treatment courses with 2103 events, representing the average patient in the SCQM population: a female seropositive, non-smoking patients with one prior b/tsDMARD, a female seropositive, non-smoking patient with one prior b/tsDMARD, mean age of 57, disease duration of 10.5 years, baseline DAS28 of 3.7, BMI of 26, who initiated her treatment before the launch of a second JAKi. (A) The median (95% CI) drug maintenance in years for the selected combination of covariates was: Tofa: 1.86 (1.52 to 2.3), OMA: 1.69 (1.48 to 2.1) and TNF: 1.32 (1.15 to 1.6) years. The multiple adjusted HRs of drug discontinuation was: HROMA versus Tofa=1.09 (0.97 to 1.24), HRTNF versus Tofa=1.29 (1.14 to1.47). The overall p-value for treatment effect was <0.001. Tofa 397 events, OMA bDMARD 689 events and TNFi 1017 events. (B) The multiple adjusted HRs of drug discontinuation due to ineffectiveness was: HRTNF versus Tofa=1.59 (1.33 to 1.89) and HROMA versus Tofa=1.19 (0.99 to 1.43). Tofa 183 events, OMA bDMARD 347 events and TNFi 580 events. (C) The multiple adjusted HRs of drug discontinuation due to intolerance and adverse events was: HROMA versus Tofa=0.74 (0.58 to 0.96), HRTNF versus Tofa=0.76 (0.59 to 0.98). Tofa 126 events, OMA bDMARD 191 events and TNFi 199 events. The difference in the total of events of panel A and the sum of events due to insufficient effectiveness or safety from panels B and C is due to discontinuation events due to ‘other’ reasons, which are not represented.
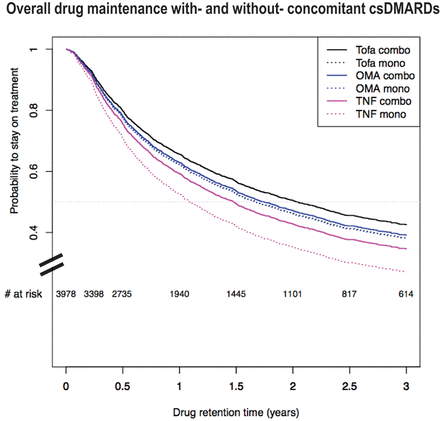
TNFi=TNF inhibitors. OMA=biologic with other modes of action (abatacept and anti-IL-6 receptor), Tofa=tofacitinib. Adjusted survival curves based on 4023 treatment courses with 2103 events, representing the average patient in the SCQM population: a female seropositive, non-smoking patients with one prior b/tsDMARD, mean age of 57, disease duration of 10.5 years, baseline DAS28 of 3.7, BMI of 26, who initiated her treatment before the launch of a second JAKi. The median (95% CI) retention time in years for this selected combination of covariates was: For TNFi: HR-MONO: 1.27 (1.08 to 1.49); for bDMARDs-OMA: HR-MONO versus COMBO: 1.03 (0.89 to 1.20); for Tofa 1.11 (0.91 to 1.35), respectively.
We further analysed the specific reasons for treatment discontinuation, based on the justification for stopping the therapy given by the treating rheumatologist. The most common reason for stopping therapy was insufficient effectiveness (57%, 50% and 46%, for TNFi, bDMARD-OMA and Tofa, respectively), followed by intolerance or adverse events (19%, 22% and 30%, for TNFi, bDMARD-OMA and Tofa, respectively). Using a competing risk analysis, we observed an approximately 50% increased risk of discontinuations due to ineffectiveness for TNFi compared with Tofa ([HR: 1.59 (95% CI 1.33 to 1.89)] and a slightly numerically increased risk for OMA-bDMARD compared with Tofa [HR: 1.19 (95% CI 0.99 to 1.43), figure 1B]. In contrast, the risk of discontinuation due to intolerance or adverse events was lower with TNFi or bDMARD-OMA compared with Tofa [HR: 0.76 (95% CI 0.59 to 0.98) and HR: 0.74 (95% CI 0.58 to 0.96, respectively)] (figure 1C).
We further examined the impact of combination therapy with csDMARDs (COMBO) on drug-maintenance. In the multivariable adjusted model, TNFi maintenance was significantly decreased in MONO [HR: 1.27 (95% CI 1.08 to 1.49)], but bDMARD-OMA and Tofa maintenance was not significantly different in MONO compared with COMBI therapy [HR: 1.03 (95% CI 0.89 to 1.20) and 1.11 (95% CI 0.91 to 1.35) respectively] (Figure 2). The full multivariable model is available as an online supplement (Table S4).
We assessed the robustness of our results by performing the same analyses as a ‘complete case’ analysis (TCs with complete baseline covariate data), which produced qualitatively similar results (Supplementary figures 3 and 4).
Secondary outcome: response rates at 1 year
In patients with a minimum follow-up time of 12 months, we analysed treatment response rates at 1 year (±3 months). Three thousand one hundred twenty-two TCs were available for analysis (1455 TNFi, 1032 bDMARD-OMA, 635 Tofa). We considered patients who had stopped their therapy within the first year as non-responders and analysed the proportion of patients in CDAI-LDA at 12 months.6 LDA was achieved in 40% of TNFi users, 46% of bDMARD-OMA users and 40% of Tofa users. The likelihood of reaching LDA at 12 months was not significantly different between the three b/tsDMARDs [OR for reaching LDA with TNFi vs Tofa: 0.90 (95% CI 0.68 to 1.18) and OR with bDMARD-OMA vs Tofa: 0.83 (95% CI 0.62 to 1.11)]. We found no evidence for effect modification by concomitant csDMARD use and the likelihood of reaching LDA with these b/tsDMARDs [TNFi: OR-MONO vs COMBO: 1.04 (95% CI 0.76 to 1.40); bDMARDs-OMA: OR-MONO vs COMBO: 1.12 (95% CI 0.80 to 1.57); Tofa: OR-MONO vs COMBO: 1.04 (95% CI 0.69 to 1.57)].
DISCUSSION
This observational study revealed generally limited overall drug maintenance for all b/tsDMARD treatment options in RA, which is explained by the patient mix and a fairly liberal access to b/tsDMARDs in Switzerland, favouring rapid treatment switching. Tofa displayed a somewhat higher overall drug maintenance compared with TNFi. Tofa users appeared to have increased discontinuation for intolerance reasons, which was offset by lower discontinuation for ineffectiveness compared with bDMARDs. Interestingly, concomitant therapy with csDMARDs did not improve the drug maintenance or the effectiveness of bDMARD-OMA or of Tofa, while TNFi appeared to require comedication with csDMARDs for optimal treatment results.
Rational therapeutic decisions live on comparisons of available treatment options. Recent years have seen an increasing number of head-to-head trials of two competing treatment options. Abatacept demonstrated similar efficacy as adalimumab in association with MTX,8 tocilizumab as monotherapy has demonstrated superior efficacy to adalimumab as monotherapy,9 JAK inhibitors demonstrated overall similar or marginally better efficacy than adalimumab in combination with MTX.10–12 A network meta-analysis combining all available randomised trials concluded that the JAKi Tofa had similar efficacy and discontinuation rates due to adverse events as bDMARDs.13 Few observational studies have compared directly the effectiveness of alternative therapeutic options, including the newer JAKi class in RA. An analysis of a US claims database found similar effectiveness rates for tofacitinib and non-TNF biologics.14 However, this analysis did not have access to disease activity outcomes and imputed effectiveness from administrative databases. Our results suggest that the overall drug maintenance of Tofa and bDMARDs-OMA is roughly comparable, while both are somewhat better the TNFi. When comparing the findings of this study with others, it is important to keep in mind that the analyses are representative for the average study population of this study sample, and may vary in different populations. We have explored specific subgroups, but found no evidence for effect modification by line of therapy or concomitant csDMARD use.
When examining the broad categories of reasons for drug discontinuation, Tofa displayed less discontinuations for ineffectiveness than the two other groups of bDMARDs, however, more for intolerance or adverse events than the two other groups of bDMARDs. A detailed analysis of the specific reasons for treatment discontinuation is needed to fully understand this potential difference, for which data are currently not available. Some differences are likely related to the modes of administration, namely between parenterally administered bDMARDs and orally administered JAKi. A significant proportion of bDMARDs are administered by nurses, which allows for regular discussion with a health professional and reassurance, which may increase adherence and mitigate treatment discontinuations for minor intolerability issues. Indeed, some observational analyses have suggested significantly higher patient adherence with bDMARDs compared with orally administered JAKi.14 Comparative research has not found differences in the rates of severe adverse events between JAK inhibitors and TNFi, with the exception of herpes zoster infections.10 13–15
Our results suggest a differential impact of combination therapy with csDMARDs with b/tsDMARDs. Concomitant use of csDMARDs is currently recommended in treatment guidelines for the management of RA for all targeted therapies.16 However, the literature suggests that the relative benefit of comedication with csDMARDs may vary according to the type of therapy. While randomised controlled studies with TNFi have generally demonstrated superiority over MTX only in combination with MTX, this has not been the case with tocilizumab or JAKi.9 17 18 An analysis from the US-based registry CORRONA suggested that the effectiveness of Tofa was not significantly decreased in monotherapy, as it was the case for TNFi [15]. In this study, drug maintenance was unaffected by concomitant csDMARDs with OMA-DMARDs and with Tofa, while TNFi seemed to be less effective when given in monotherapy. While we need to acknowledge that part of this effect may be attributed to unmeasured confounding, we believe that the relative effect of unmeasured confounding is unlikely to differ between the different groups of therapies. To account for the potential confounding effect of an increasing number of available therapeutic alternatives, we adjusted the analysis for calendar time. While we could confirm a trend for decreasing drug maintenance over time with all b/tsDMARDs, we observed a steeper decrease in overall drug maintenance for Tofa after baricitinib became available, suggesting some switching among JAKi, probably for non-medical reasons.
This study has limitations. Missing data and incomplete follow-up is an issue in most registries; however, our primary outcome, drug retention, is a fairly robust outcome in this setting. All analyses were performed both with and without multiple imputations for missing baseline covariates. The results of complete case analysis (Figure S4). yielded results consistent with analyses using multiple imputations and did not change the interpretations, suggesting that missing data points did not bias our results. Our effectiveness analysis at 1 year is limited by a reduced proportion of patients having an available disease activity assessment (CDAI) around 1 year. We used prediction (mixed-effects models) to attempt to recover the missing information; however, the response rates at 1 year need to be interpreted with caution. As with all non-randomised studies, confounding by indication may occur if the choice of a particular drug is linked to the outcome. To limit the risk of confounding by indication, we restricted the analysis to a time period when all treatment options were available, and adjusted the analysis by line of therapy and other potential confounding factors. Nevertheless, observational studies may be confounded by unmeasured factors. The strength of this observational study relies on the wide variety of RA patients, representative of the ‘real world’ and the statistical power stemming from a relatively large number of patients.
In order to help clinicians and their patients to choose among the ever-increasing armentarium of second-line antirheumatic therapies and pick the treatment most likely to provide benefit in their particular situation, more comparative effectiveness research in large observational registries are useful. The results of this study suggest that ‘real-world’ drug maintenance and effectiveness of available therapeutic options for second-line or third-line treatment of RA are generally comparable. The newer class of JAKi, represented in this analysis by Tofa, had somewhat increased overall drug maintenance compared with TNFi and similar effectiveness to other bDMARDs, suggesting that Tofa is a valuable alternative to available treatment options in RA. Our results also suggest that the relative benefit of concomitant csDMARDs varies according to the type of targeted DMARDs, with mainly TNFi requiring combination with csDMARDs for optimal responses.
Acknowledgments
All contributions to the SCQM cohorts are on a completely voluntary, without any financial compensation by the SCQM. We thank all contributing institutions and all patients for their efforts in collecting these data. A list of rheumatology offices and hospitals that are contributing to the SCQM registries can be found on www.scqm.ch/institutions.
REFERENCES
Footnotes
Contributors I, the submitting author has the right to grant and does grant on behalf of all authors of the work (as defined in the below author licence), an exclusive licence and/or a non-exclusive licence for contributions from authors who are: (i) UK Crown employees; (ii) where BMJ has agreed a CC-BY licence shall apply and/or (iii) in accordance with the terms applicable for US Federal Government officers or employees acting as part of their official duties; on a worldwide, perpetual, irrevocable, royalty-free basis to BMJ Publishing Group Ltd (‘BMJ’) its licensees and where the relevant Journal is co-owned by BMJ to the co-owners of the journal, to publish the work in this journal and any other BMJ products and to exploit all rights, as set out in our licence. The submitting author accepts and understands that any supply made under these terms is made by BMJ to the submitting author unless you are acting as an employee on behalf of your employer or a postgraduate student of an affiliated institution which is paying any applicable article publishing charge (‘APC’) for Open Access articles. Where the submitting author wishes to make the work available on an Open Access basis (and intends to pay the relevant APC), the terms of reuse of such Open Access shall be governed by a Creative Commons licence – details of these licences and which Creative Commons licence will apply to this work are set out in our licence referred to above. Other than as permitted in any relevant BMJ Author’s Self Archiving Policies, I confirm this work has not been accepted for publication elsewhere, is not being considered for publication elsewhere and does not duplicate material already published. I confirm all authors' consent to publication of this work and authorise the granting of this licence.
Funding The SCQM is financially supported by pharmaceutical industries and private donors. A list of financial supporters can be found on www.scqm.ch/sponsors. An Investigator-Initiated Research Grant was provided by Pfizer to the Swiss Clinical Quality Management in Rheumatic Diseases (SCQM) Foundation for a prospective observational study and development of this manuscript and to Pascal Zufferey, an institution investigator at the SCQM Foundation. A separate financial support from Pfizer was received by Pr. Axel Finckh, an employee of the Geneva University Hospital (HUG), for a distinct analysis in connection with analyses of the SCQM database and the development of this manuscript. The results of both analyses are included in this manuscript.
Competing interests Yes, there are competing interests for one or more authors and I have provided a Competing Interests statement in my manuscript.
Patient consent for publication Not required.
Ethical approval information This project was approved by the Geneva Ethical Committee (Protocole GE 10-089) on 31st of March 2015.
Provenance and peer review Not commissioned; externally peer reviewed.